BASE COMPOSITIONS FOR PREPARING SURFACTANT FREE TOPICAL COMPOSITIONS
FIELD OF THE INVENTION The present invention relates to base compositions for preparing surfactant free topical, oral, nasal, anal, ophthalmic, or vaginal compositions and a method for preparing such surfactant free compositions.
BACKGROUND OF THE INVENTION Most topical preparations currently sold contain a wide variety of physiologically active agents ("active agents") and/or aesthetic modifying agents. Physiologically active agents are compounds which cause a physical change to the body following their application. Examples of such agents include alpha hydroxy acids, antioxidants, and vitamins. Aesthetic modifying agents provide the preparation with a desired physical characteristic such as, for example, the degree of moisturization, oil content, and physical form of the preparation. Some examples of aesthetic modifying agents include silicone fluids and derivatives thereof, waxes, botanical (vegetable) oils, hydrocarbon-based oils, esters and fragrances.
The performance of these active agents is dependent upon the vehicle used to deliver them. These vehicles range from simple solvents, such as water and ethanol, to complex emulsions. Unfortunately not all active agents are completely soluble or compatible with all vehicles. For example, oil soluble active agents are typically not compatible with water or water- based gel vehicles. As a result, many such products exhibit poor delivery of active agents, have poor tactile properties, or are thermodynamically unstable and result in a commercially unacceptable shelf life. Topical preparations having a non-water based solvent do not usually have an aesthetically pleasing appearance, feel, and/or fragrance. Furthermore, non- water based solvents
can cause unwanted side effects, such as irritation or damage to the epithelial surface to which they are applied.
To avoid the problems associated with non-water based solvents, stable emulsions are commonly employed to deliver physiologically active agents and aesthetic modifying agents. These emulsions form either spherical micelles of one or more hydrophobic liquid materials in water or spherical droplets of water in a hydrophobic fluid.
Such emulsions are typically formed by separately preparing an oil phase and a water phase, hi other words, the hydrophobic ingredients are dissolved in a suitable oil phase and the hydrophilic ingredients are dissolved in water. The two phases are heated to at least 70° C and mixed together. Emulsifying agents are usually added to the mixture in order to reduce the surface tension between the oil and water phases, thereby making the combination of the two phases more stable. The mixture is then slowly cooled to ensure the formation of crystalline structures which enhance the stability of the emulsion. Emulsions prepared by this method usually have a homogeneous opaque white appearance and a smooth or pleasant feeling upon application to the skin or other epithelial surface.
A significant drawback of emulsions is their use of surfactants. Surfactants can strip the material lipid barrier of the skin and the lipid bilayer of epithelial cell membranes leaving tissue vulnerable and irritating the skin. The damaged barrier permits the passage of other materials that can cause irritation or increase skin sensitivity and allergic reactions. The literature is replete with clinical evidence of the damaging consequences that can occur with the use or overuse of surfactants. For example, Effendy I., Maibach H.I., "Surfactants and experimental irritant contact dermatitis", Contact Dermatitis, 33(4):217-25 (10/1995), indicates that "[m]any surfactants elicit irritant reactions when applied to the skin, partially due to their relative ability to solubilize lipid membranes." InBaranyE., LindbergM., LodenM., "Biophysical characterization of skin damage and recovery after exposure to different surfactants", Contact Dermatitis 40(2):98-103 (2/1999), the authors states that "[t]he majority of adverse skin reactions to personal care products are presumed to be caused by irritant substances, like surfactants."
Additionally, lowering the surface tension of a topical preparation generally increases the surface exposure of the active agent or aesthetic modifying agent to oxygen and other destabilizing materials. For example, in a topical preparation containing retinol as an
active ingredient, the instability of the preparation may decrease the efficacy of the retinol. The instability of an unsaturated fatty acid as an aesthetic modifying agent leads to color changes in the preparation and malodor.
Other drawbacks to emulsions include: (1) Many materials having aesthetic properties, such as, for example, fluorinated compounds, are not easily incorporated into emulsions. Aesthetic properties relate to the way a material feels including the material's texture, oil content, water content, etc.
(2) Each time the oil or water phase is changed in an emulsion, the amounts and types of emulsifying agents in the emulsion needs to be readjusted; and (3) Many active agents and aesthetic modifying agents degenerate during the preparation of an emulsion, for example, during heating, and after the emulsion has been formed.
Since the time between manufacturing and sale of a cosmetic product is typically several weeks, the product is often no longer "fresh" or effective since the active agent has degenerated or deteriorated. To offset instability problems, many other materials such as chelating agents, antioxidants and masking agents are usually included in the product.
Typical emulsions are time consuming to prepare, require heating, are produced in multiple phases, are slow cooling, and often require high shear conditions to get the particle size small enough for maximum stability. Larger batches may require 8 to 24 hours to process and can take several days to set up. It is also often difficult to control the process parameters for preparing the emulsion. If any factors such as the heating, cooling or mixing rates are not carefully duplicated, the preparation may have different properties than the preceding batches of the same product. As a result, the stability of the emulsion may vary from batch to batch. Often the difference of a single parameter is significant enough to cause the product to be outside the established optimum specifications. These batches then have to be either discarded or re-worked. The lack of reproducibility is especially problematic when the product contains a physiologically active agent. Lack of reproducibility can effect product performance and end user satisfaction. The lack of reproducibility also results in products having different aesthetic properties which the end user will perceive as a lack of quality and will ultimately lead to consumer dissatisfaction or reduced compliance.
Emulsions are typically expensive to manufacture. This is due to a variety of factors including the energy to heat the batch, the specialized equipment required to process the emulsion, such as specialized pumps and cooling/heating equipment, and the time the process ties up equipment and personnel. Moreover, such emulsions cannot be easily processed or customized at the point of purchase. Since most skin care preparations are prepackaged and have predetermined dosages, dermatologists cannot readily administer to patients varying dosages of an active agent. As a result, a patient may need to apply two or more different skin care preparations since a single preparation with all of the prescribed active agents may not be available.
Some dermatologists prepare their own skin care preparations. These skin care preparations typically have poor aesthetic properties resulting in poor patient compliance. Thus, it would be desirable for dermatologists to be able to quickly and easily prepare skin care preparations having varying dosages of active agents and an aesthetically pleasing appearance.
Present cosmetic products contain predeteπnined amounts of active agents.
Customers cannot pick and choose which ingredients to include in these products. Many customers do not purchase certain cosmetic products because of an allergic reaction with one or more of the ingredients included in the product. For example, many customers are allergic to various fragrances. It would therefore be advantageous to prepare the cosmetic product at the point of sale without the fragrances. Also, customers may have to apply two or more different cosmetic products to get a desired effect since a single product with the desired combination of active agents and/or aesthetic modifying agents may not be on the market. Many cosmetic products are sold in only one form, such as a spray, gel or lotion. Customers, however, may prefer other forms of the cosmetic product.
Prior to the present invention, it was not practical to prepare custom cosmetic products at the point of sale. The preparation of most current cosmetic products require heating, other energy expensive processes, and or large industrial equipment. As a result, it was not economically feasible to prepare custom cosmetic products at the point of sale. Furthermore, active ingredients which are heat sensitive and oil soluble could not readily be incorporated into cosmetic products by conventional heating without partially or completely degrading the active ingredient.
For the foregoing reasons, there is a need for a substantially surfactant free cosmetic product which can be prepared at the point of sale. Also, there is a need for a method of preparing such a cosmetic product which is fast and does not require heating or other expensive processing techniques.
SUMMARY OF THE INVENTION
The present inventors have discovered a base composition which when mixed with one or more dispersions of hydrophobic ingredients, and in particular cationic dispersions, forms a highly stable composition which is substantially free of aggregates and in which the water and hydrophobic ingredients in the composition do not readily separate. The compositions formed maybe topically, orally, nasally, anally, ophthalmically, or vaginally applied to an animal, such as a human. The base composition comprises (a) aphosphorylated starch derivative; (b) one or more co-thickening agents, such as carbomer and acrylate/alkyl acrylate crosspolymers; and (c) water.
Another embodiment of the invention is a composition for topical, oral, nasal, anal, ophthalmic, or vaginal application comprising the base composition of the present invention and at least one dispersion comprising suspended particles of a hydrophobic active agent, a hydrophobic adjuvant, or a combination thereof. The composition is substantially free of emulsifying surfactants and the suspended particles have a diameter less than about 500 nm. Preferably, the composition comprises at least one cationic dispersion. Yet another embodiment is a method of preparing a composition for topical, oral, nasal, anal, ophthalmic, or vaginal application. The method comprises mixing (a) the base composition of the present invention, and (b) at least one dispersion comprising suspended particles of a hydrophobic active agent, a hydrophobic adjuvant, or a combination thereof. The composition prepared is substantially free of emulsifying surfactants and the suspended particles have a diameter less than about 500 nm. Mixing may be performed with a propeller mixer or manually, i.e., by hand. Preferably, the base composition is premanufactured. According to one preferred embodiment, the composition prepared contains at least one cationic dispersion. Since the composition is simple and quick to prepare, custom cosmetic compositions may be prepared at the point of sale for customers in minutes. Prior to the present invention, such products would take hours to be prepared.
DETAILED DESCRIPTION OF THE INVENTION
The present invention relates to a base composition for preparing a substantially surfactant free composition for topical, oral, nasal, anal, ophthalmic, or vaginal application, the base composition comprising (a) a phosphorylated starch derivative; (b) one or more co- thickening agents, such as carbomer and acrylates/alkyl acrylate crosspolymers; and (c) water. The base composition may be prepared by methods known in the art. The term "phosphorylated starch derivative" includes, but is not limited to, starches containing a phosphate group. Suitable phosphorylated starch derivatives include, but are not limited to, hydroxyalkyl starch phosphates, hydroxyalkyl distarch phosphates, and any combination of any of the foregoing. Non-limiting examples of hydroxyalkyl starch phosphates and, hydroxyalkyl distarch phosphates include hydroxyethyl starch phosphate, hydroxypropyl starch phosphate, hydroxypropyl distarch phosphate, and any combination of any of the foregoing. According to a preferred embodiment, the base composition comprises a hydroxyalkyl distarch phosphate and more preferably hydroxypropyl distarch phosphate.
Co-thickening Agents
Suitable co-thickening agents include, but are not limited to, carbohydrate based thickening agents, polymeric and copolymeric thickening agents, inorganic thickening agents, protein thickening agents, polypeptide thickening agents, and any combination of any of the foregoing.
Non-limiting examples of suitable carbohydrate based thickening agents include algin and derivatives and salts thereof, such as algin, calcium alginate, propylene glycol alginate, and ammonium alginate; carrageenan (Chondrus crispus) and derivatives and salts thereof, such as calcium carrageenan and sodium carrageenan; agar; cellulose and derivatives thereof, such as carboxymethyl hydroxyethylcellulose, cellulose gum, cetyl hydroxyethylcellulose, hydroxyethylcellulose, hydroxypropylcellulose, hydroxypropyl methylcellulose, methylcellulose, ethylcellulose, and cellulose gum; chitosan and derivatives and salts thereof, such as hydroxypropyl chitosan, carboxymethyl chitosan, and chitin; gellan gum; guar (Cyamopsis tetragonoloba) and derivatives thereof, such as guar hydroxypropyltrimomum chloride and
hydroxypropyl guar; hyaluronic acid and derivatives thereof; dextran and derivatives thereof; dextrin; locust bean (Ceratonia siliqua) gum; mannans and derivatives thereof, such as C1-5 alkyl galactomannan; starches, such as starch polyacrylonitrile copolymer-potassium salt and starch polyacrylonitrile copolymer-sodium salt; pectin; sclerotium gum; tragacanth (Astragalus gummifer) gum; xantham gum and derivatives thereof; and any combination of any of the foregoing.
Non-limiting examples of suitable polymeric and copolymeric thickening agents include acrylates, methacrylates, polyethylene and derivatives thereof, and any combination of any of the foregoing. Suitable acrylates and methacrylates include, but are not limited to, carbomer and derivatives and salts thereof, acrylate/Cιo-C3o alkyl acrylate crosspolymer, acrylate/ceteth-20 itaconate copolymer, acrylate/ceteth-20 methacrylate copolymers, acrylate/steareth-20 methacrylate copolymers, acrylate/steareth-20 itaconate copolymers, acrylate/steareth-50 acrylate copolymers, acrylate VA crosspolymers, acrylate/vinyl isodecanoate crosspolymers, acrylic acid/acrylonitrogen copolymers, ammonium acrylate/acrylonitrogen copolymers, glyceryl polymethacrylate, polyacrylic acid, PNM MA decadiene crosspolymer, sodium acrylate/vinyl isodecanoate crosspolymers, sodium carbomer, ethylene/acrylic acid copolymer, ethylene/NA copolymer, acrylate/acrylamide copolymer, acrylate copolymers, acrylate/hydroxyester acrylate copolymers, acrylate/octylarylamide copolymers, acrylate/PNP copolymers, AMP/acrylate copolymers, butylester of PNM/MA copolymer, carboxylate vinylacetate terpolymers, diglycol/CHDM/isophthalates/SIP copolymer, ethyl ester of PNM/MA copolymer, isopropyl ester of PVM/MA copolymer, octylacrylamide/acrylate/butylaminoethyl methacrylate copolymers, polymethacrylamidopropyltrimonium chloride, propylene glycol oligosuccinate, polyvinylcaprolactam, PNP, PVP/dimethylaminoethylmethacrylate copolymer, PNP/DMAPA acrylate copolymers, PNP/carbamyl polyglycol ester, PNP/NA copolymer, PVP/NA vinyl propionate copolymer, PNP/vinylcaprolactam DMAPA acrylate copolymers, sodium polyacrylate, NA/butyl maleate/isobomyl acrylate copolymers, NA/crotonates copolymer, NA/crotonates vinyl neodecanoate copolymer, NA crotonates/vinyl propionate copolymer, vinyl caprolactarn/PNP/dimetxylammoethylmethacrylate copolymer, and any combination of any of the foregoing. The following acronyms relate to: AMP = aminomethyl propanol; DMAPA = dimethylammopropylamine; NA = vinyl acrylates; PNM/MA = copolymer of methyl vinyl ether
and maleic anhydride; CHDM = 1,4-cycolhexanedimethanol; SIP = sulfoisophthalic acid; PNP = polymer of l-vinyl-2 pyrrolidine monomers.
Νon-limiting examples of suitable inorganic thickening agents include clays and derivatives thereof, silicates, silicas and derivatives thereof, and any combination of any of the foregoing. Suitable clays and derivatives thereof include, but are not limited to, bentonite and derivatives thereof, such as quaternium-18 bentonite; hectorite and derivatives thereof, such as quaternium- 18 dectorite; montmorillonite; and any combination of any of the foregoing. Suitable silicates include, but are not limited to, magnesium aluminum silicate, sodium magnesium silicate, lithium magnesium silicate, tromethamine magnesium aluminum silicate, and any combination of any of the foregoing. Suitable silicas and derivatives thereof include, but are not limited to, hydrated silica, hydrophobic silica, and any combination of any of the foregoing.
Suitable protein and polypeptide thickening agents include, but are not limited to, proteins and derivatives and salts thereof, polypeptides and derivatives and salts thereof, and any combination of any of the foregoing. Νon-limiting examples of protein and polypeptide thickening agents include albumin, gelatin, keratic and derivatives thereof, fish protein and derivatives thereof, milk protein and derivatives thereof, wheat protein and derivatives thereof, soy protein and derivatives thereof, elastin and derivatives thereof, silk protein and derivatives thereof, and any combination of any of the foregoing. A preferred sunscreen agent is a mixture of ethylhexyl methoxycinnamate, butyl methoxydibenzoylmethane, cyclomethicone, phospholipids, and water, and is available as Solarease® from Collaborative Laboratories, Inc. of East Setauket, New York.
Preferred thickening agents include, but are not limited to, carbomer, acrylate/alkyl acrylate crosspolymers, acrylate/vinyl isododecanoate crosspolymer, xantham gum, locust bean gum, guar gum, and any combination of any of the foregoing. A more preferred combination of thickening agents comprises carbomer and an acrylate/alkyl acrylate copolymer, such as an acrylate/C10-C3o alkyl acrylate copolymer. According to the International Cosmetic Ingredient Dictionary and Handbook (7th Ed., The Cosmetic, Toiletry, and Fragrance Association), carbomer is a homopolymer of acrylic acid crosslinked with an allyl ether of pentaerythritol, an allyl ether of sucrose, or an allyl ether of prόpylene. The term "acrylate/alkyl acrylate crosspolymer" includes, but is not limited to, copolymers of alkyl acrylates with one or more monomers of
acrylic acid, methacrylic acid, or one of their short chain (i.e. CM alcohol) esters, wherein the crosslinking agent is, for example, an allyl ether of sucrose or pentaerytritol. Preferably, the alkyl acrylates are C10-C o alkyl acrylates. Examples of such copolymers include, but are not limited to, those commercially available as Carbopol™ 1342, Carbopol™ 1382, Pemulen™ TR-1, and Pemulen™ TR-2, from Goodrich Specialty Chemicals of Cleveland, OH.
The weight ratio of phosphorylated starch derivative to co-thickening agents generally ranges from about 10:1 to about 1:10 and preferably from about 8:1 to about 1:8, and more preferably from about 5:1 to about 1:5. The base composition typically comprises from about 1 to about 10% and preferably from about 4 to about 7% and more preferably from about 2 to about 5% by weight of phosphorylated starch derivative. The base composition typically comprises from about 0.1 to about 10 % preferably from about 0.8 to about 8% and more preferably from about .5 to about 5% by weight of co-thickening agents.
According to a preferred embodiment, the weight ratio of hydroxyalkyl starch or distarch phosphate to carbomer in the base composition generally ranges from about 1 : 1 to about 20:1. The weight ratio preferably ranges from about 2:1 to about 10:1 and more preferably is about 5:1. The weight ratio of hydroxyalkyl starch or distarch phosphate to acrylate/alkyl acrylate copolymer generally ranges from about 1:1 to about 16:1. The weight ratio preferably ranges from about 2:1 to about 8:1 and more preferably is about 4:1. Typically, this preferred base composition comprises from about 1 to about 10% by weight of hydroxyalkyl starch phosphate or hydroxyalkyl distarch phosphate; from about 0.15 to about 3% by weight of carbomer; and from about 0.25 to about 5% by weight of an acrylate/alkyl acrylate copolymer, based upon 100% total weight of base composition. Preferably, the base composition comprises from about 2 to about 5% by weight of hydroxyalkyl starch phosphate or hydroxyalkyl distarch phosphate; from about 0.3 to about 1.5%) by weight of carbomer; and from about 0.5 to about 3%> by weight of an acrylate/alkyl acrylate copolymers, based upon 100% total weight of base composition. According to a preferred embodiment, the base composition comprises about 3%> by weight of hydroxypropyl distarch phosphate; about 0.6%> by weight of carbomer; and about 0.75%o by weight of an acrylate/ o- o alkyl acrylate crosspolymer, based upon 100% total weight of base composition.
Another embodiment of the invention is a composition for topical, oral, nasal, anal, ophthalmic, or vaginal application comprising the base composition of the present invention and at least one dispersion comprising suspended particles of a hydrophobic active agent, a hydrophobic adjuvant, or a combination thereof. The composition is substantially free of emulsifying surfactants and the suspended particles have a diameter less than about 500 nm. Typically, besides the base composition, the composition for application only contains water- soluble ingredients and/or dispersions of hydrophobic active agents and or hydrophobic adjuvants. Generally, hydrophobic ingredients which are not in the form of a dispersion are not included in the composition, or at least not in any substantial amounts. Preferably, the composition contains at least one cationic dispersion.
The composition and base composition preferably are free of alginates and polyquaternium and derivatives thereof. The inclusion of alginates often result in an aestetically unpleasing product.
Generally, the composition contains from about 0.01 to about 35% by weight, preferably from about 0.4 to about 10% by weight, and more preferably from about 0.4 to about 6% by weight of the base composition, based upon 100% weight of total composition. The composition preferably has a pH greater than 5 and more preferably greater than 6.
The composition for topical, oral, nasal, anal, ophthalmic, or vaginal application may be prepared by mixing (a) the base composition of the present invention, and (b) at least one dispersion comprising suspended particles of a hydrophobic active agent, a hydrophobic adjuvant, or a combination thereof.
Other active agents, aesthetic modifying agents, and adjuvants, such as those described in Remington's Pharmaceutical Sciences, 19th Edition, A. R. Gennaro (1995) and the International Cosmetic Ingredient Dictionary and Handbook, 7* Edition (1997), published by The Cosmetic, Toiletry, and Fragrance Association (both of which are hereby incorporated by reference), may be incorporated into the composition. Generally, all hydrophobic ingredients to be included in the final composition are added as dispersions (i.e. a dispersion of the hydrophobic ingredient is prepared before it is mixed with the base composition and the dispersion).
The composition prepared is substantially free of emulsifying surfactants and the suspended particles have a diameter less than about 500 nm. The composition preferably
comprises less than about 3%> by weight and more preferably less than about 1% by weight of emulsifying surfactants, based upon 100%o weight of total composition.
The composition is substantially free of aggregates. Furthermore, the water and hydrophobic ingredients in the composition do not readily separate. The composition may be a cream, gel, or lotion, serum or spray.
Preferably, the base composition is premanufactured, i.e., prepared at a location remote from where the mixing step is performed or prepared in large quantities. The term "large quantities" is herein defined as a quantity greater than that needed to produce a single final product and is preferably many multiples times that. The base composition is typically premanufactured in large batches.
Mixing is generally performed at a temperature of from about 15 to about 30° C, preferably at a temperature of from about 20 to about 30° C, and most preferably at ambient temperature. Since the hydrophobic active agent or hydrophobic adjuvant is added to the base composition as a dispersion, heating and other expensive processing steps are not required to obtain a homogenous final composition. Preferably, the composition is not heated.
The dispersion is generally a homogenous fluid which is stable for a commercially relevant period of time. The dispersion typically remains stable for at least 2 weeks and preferably at least 2 months.
According to a preferred embodiment, the dispersion is prepared by mixing from about 0.1%) to about 70% by weight of hydrophobic active agent and/or hydrophobic adjuvant with from about 30% to about 99.9% by weight of aqueous phase under high pressure and high shear conditions, based upon 100% weight of total dispersion. Suitable high pressure and high shear dispersions are sold under the tradename SanSurf® by Collaborative Laboratories, Inc. of East Setauket, NY. The aqueous phase contains water and, optionally, other hydrophilic adjuvants. More preferably, the mixing is performed with shearing at a pressure of from about 9,000 to about 25,000 psi to form a dispersion having an average particle size ranging from about 50 to about 500 nm.
Hydrophobic Active Agent or Hydrophobic Adjuvant Dispersion
A hydrophobic active agent or hydrophobic adjuvant is an active agent or adjuvant which has a non polar property which makes it essentially insoluble in water or water and polar solvent solution. Hydrophobic active agents and hydrophobic adjuvants of the present invention include, but are not limited to, partially and fully hydrophobic active agents and partially and fully hydrophobic adjuvants. For example, hydrophobic active agents encompassed by the present invention include compounds and complexes which contain a hydrophobic moiety.
The composition of the present invention may also include non-hydrophobic active agents and non-hydrophobic adjuvants.
The dispersion containing the suspended particles generally contains from about 0.01 to about 70%> by weight of oil, based upon 100%> weight of total dispersion. Preferably, the dispersion contains from about 1.0 to about 50% by weight of oil, based upon 100%> weight of total dispersion. The oil component of the composition may include active agents and adjuvants which are oils.
The dispersion is a suspension of liquid or solid particles of colloidal size or larger in a liquid medium. Generally, the dispersion contains suspended particles, such as oil particles (or oil droplets), having a diameter less than about 500 nm. The diameter of the suspended particles preferably ranges from about 50 nm to about 500 nm and more preferably from about 250 to about 500 nm. Preferably, the oil droplets contain one or more lipophilic materials. The oil droplets may have a charge as determined by zeta potential measurements. The oil droplets may be prepared by ultra high shear mixing or microfluidization. Preferred oil containing dispersions are sold under the tradename SanSurf® by Collaborative Laboratories, Inc. of East Setauket, NY, and Dermasomes™ by Microfluidics Corp. of Newton, MA.
Active Agents
Suitable active agents include, but are not limited to, anti-acne agents, antimicrobial agents, anti-inflammatory agents, analgesics, antierythemal agents, antipruritic agents, antiedemal agents, antipsoriatic agents, antifungal agents, skin protectants, sunscreen agents, vitamins, antioxidants, scavengers, antiirritants, antibacterial agents, antiviral agents, antiaging agents, protoprotection agents, hair growth enhancers, hair growth inhibitors, hair removal agents, antidandruff agents, anti-seborrheic agents, exfoliating agents, wound healing agents, anti-ectoparacitic agents, sebum modulators, immuno odulators, hormones, botanicals, moisturizers, astringents, cleansers, sensates, antibiotics, anesthetics, steroids, tissue healing substances, tissue regenerating substances, amino acids, peptides, minerals, ceramides, biohyaluronic acids, and any combination of any of the foregoing.
Preferred anti-acne agents include, but are not limited to, salicylic acid, retinoic acid, alpha hydroxy acid, benzyl peroxide, sodium sulfacetamide, clindamycin, and any combination of any of the foregoing. Preferred combinations of anti-acne agents to be incorporated in the composition include salicylic acid, retinoic acid, and hydrocortisone; sodium sulfacetamide and clindamycin; salicylic acid and clindamycin; salicylic acid, alpha hydroxy acid, and tetrahydrozoline.
Suitable antimicrobial agents include, but are not limited to, benzalkonium chloride, benzethonium chloride, chlorhexidine gluconate, chloroxylenol, cloflucarban, fluorosalan, hexachlorophene, hexylresorcinol, iodine complex, iodine tincture, para- chloromercuriphenol, phenylmercuric nitrate, thimerosal, vitromersol, zyloxin, triclocarban, triclosan, methyl-benzethonium chloride, nonyl phenoxypoly(ethyleneoxy) ethanol-iodine, para- chloro-meta-xylenol, providone-iodine complex, poloxamer-iodine complex, triclorcarban, undecoylium chloride-iodine complex, and any combination of any of the foregoing. Suitable antiinflammatory agents include, but are not limited to, alidoxa, allantoin, aloe vera, aluminum acetate, aluminum hydroxide, bismuth subnitrate, boric acid, calamine, casein, cellulose, microporous, cholecatciferol, cocoa butter, cod liver oil, colloidal oatmeal, cystein hydrochloride, dexpanthenol, dimethicone, glycerin, kaolin, lanolin, live yeast cell derivative, mineral oil, peruvian balsam, petrolatum, protein hydrolysate, racemetliionine, shark liver oil, sodium bicarbonate, sulfur, talc, tannic acid, topical starch, vitamin A, vitamin E, white
petrolatum, zinc acetate, zinc carbonate, zinc oxide, hydrocortisone, betamethasone, ibuprofen, indomethicin, acetyl salicylic acid, tacrolimus, flucoinolone acetonide, sodium sulfacetamide, and any combination of any of the foregoing.
Suitable analgesics include, but are not limited to, diphenhydramine, tripeiennamme, benzocaine, dibucaine, lidocaine, tetracaine, camphor, menthol, phenol, resorcinol, matacresol, juniper tar, methylsalicylate, turpentine oil, capsicum, methyl nicotinate, b-glucan, and any combination of any of the foregoing.
Suitable antierythemal agents include, but is not limited to, tetrahydrozoline and hydracortisone. Suitable antipruritic agents include, but are not limited to, benadryl, pramoxine, antihistamines, and any combination of any of the foregoing.
Suitable antiedemal agents, include, but are not limited to, pregnenalone acetate, tanin glyrosides, and any combination of any of the foregoing.
Suitable antipsoriatic agents include, but are not limited to, caleipotriene, coal tar, anthralin, vitamin A, and any combination of any of the foregoing. Preferred combinations of antipsoriatic agents include, but are not limited to, hydrocortisone, retinoic acid, and alpha hydroxy acid; dovonex, salicylic acid, and a sunscreen agent; indomethicin, salicylic acid, and urea; anthralin and salicylic acid; and anthralin and indomethicin. Other suitable antipsoriatic agents include, but are not limited to, caleipotriene, coal tar, anthralin, vitamin A, and any combination of any of the foregoing.
Suitable antifungal agents include, but are not limited to, clioquinol, haloprogin, miconazole nitrate, clotrimazole, metronidazole, tolnaftate, undecylenic acid, iodoquinol, and any combination of any of the foregoing.
Suitable skin protectants include, but are not limited to, cocoa butter, dimethicone, petrolatum, white petrolatum, glycerin, shark liver oil, allantoin, and any combination of any of the foregoing.
Suitable sunscreen agents include, but are not limited to, ethylhexyl methoxycinnamate, avobenzone, benzoρhenone-3, octacrylene, titanium dioxide, zinc oxide, and any combination of any of the foregoing. A preferred sunscreen agent is a mixture of ethylhexyl
methoxycinnamate, butyl methoxydibenzoylmethane, cyclomethicone, phospholipids, and water, and is available as Solarease® from Collaborative Laboratories, Inc. of East Setauket, New York. Suitable antioxidants include, but are not limited to, scavengers for lipid free radicals and peroxyl radicals, quenching agents, and any combination of any of the foregoing. Suitable antioxidants include, but are not limited to, tocopherol, BHT, beta carotene, vitamin A, ascorbic acid, ubiquinol, ferulic acid, azelaic acid, thymol, catechin, sinapic acid, EDTA, lactoferrin, rosmariquinone, hydroxytyrosole, sesamol, 2-tMoxanthine, nausin, malvin, carvacone, chalcones, glutathione isopropyl ester, xanthine, melanin, guanisone, lophorphyrins, 8- hydroxyxanthine, 2-thioxanthione, vitamin B1 , plant alkaloids, catalase, quercetin, tyrosine, SOD, cysteine, methionine, genistein, NDG, procyanidin, hamamelitannin, ubiquinone, trolox, licorice extract, propyl gallate, sinapic acid, and any combination of any of the foregoing. Suitable vitamins include, but are not limited to, vitamin E, vitamin A palmitate, vitamin D, vitamin F, vitamin B6, vitamin B3> vitamin B1 j vitamin C, ascorbyl palmitate, vitamin E acetate, biotin, niacin, DL-panthenol, and any combination of any of the foregoing. A preferred sunscreen agent is a mixture of ethylhexyl methoxycinnamate, butyl methoxydibenzoylmethane, cyclomethicone, phospholipids, and water, and is available as Solarease® from Collaborative Laboratories, Inc. of East Setauket, NY.
Suitable amino acids include, but are not limited to, glycine, serine, and any combination of any of the foregoing.
Aesthetic Modifying Agents
The composition preferably includes at least one aesthetic modifying agent. An aesthetic modifying agent is a material which imparts desirable tactile, olfactory, taste or visual properties to the surface to which the composition is applied. The aesthetic modifying agent may be hydrophobic or hydrophilic. The aesthetic modifying agent is preferably hydrophobic and is more preferably an oil, wax, solid or paste.
A dispersion of one or more hydrophobic aesthetic modifying agents is preferably prepared before the hydrophobic aesthetic modifying agents are incorporated into the composition. The hydrophobic aesthetic modifying agents may be dispersed into an aqueous phase by methods known in the art, such as by ultra high shear mixing and microfluidization.
The final composition may be prepared by mixing the dispersions containing the hydrophobic aesthetic modifying agents with the base composition and any other adjuvants.
Since the hydrophobic aesthetic modifying agents are added to the base composition as dispersions, heating *and other expensive processing steps are not required to obtain a homogenous final composition.
An example of an aesthetic modifying agent is a mono, di, tri or poly alkyl ester or ether of a di, tri, or polyhydroxy compound, such as ethylene glycol, propylene glycol, glycerin, sorbitol or other polyol compound. Examples of such esters and ethers include, but are not limited to, saturated and unsaturated, linear and branched vegetable oils, such as soybean oil, babassu oil, castor oil, cottonseed oil, Chinese tallow oil, crambe oil, perilla oil, danish rapeseed oil, rice bran oil, palm oil, palm kernel oil, olive oil, linseed oil, coconut oil, sunflower oil, safflower oil, peanut oil and corn oil. Preferred saturated and unsaturated vegetable oils are those having fatty acid components with 6 to 24 carbon atoms. A more preferred vegetable oil is soybean oil. An example of a hydrophobic aesthetic modifying agent is a compound having the formula CnH(2n+2.m) where n is an integer greater than or equal to 6 and m is 0 or an even integer no greater than n. Such compounds include, but are not limited to, saturated and unsaturated, linear, branched, and cyclic hydrocarbon chains. Preferred examples of such compounds include, but are not limited, mineral oil, petrolatum, permethyl fluids, polybutylenes, and polyisobutylenes.
Another example of a hydrophobic aesthetic modifying agent has the formula
R, -O- -R?
O
or the formula
R i C O (CH2)n C O- -R*
O O
where Ri is a saturated or unsaturated, linear, branched or cyclic Ci-C24 alkyl; R2 is hydrogen or a saturated or unsaturated, liner, branched or cyclic Ci-C24 alkyl; and n is an integer from 0 to 20. Examples of such aesthetic modifying agents include, but are not limited to, isopropyl palmitate and diisopropyl adipate. Yet another aesthetic modifying agent is silicone. Silicone may provide lubrication and/or shine to the composition. Preferably, the silicone is insoluble in water. Suitable water-insoluble silicone materials include, but are not limited to, polyalkylsiloxanes, polyarylsiloxanes, polyalkylarylsiloxanes, polysiloxane gums andpolyethersiloxane copolymers. Examples of suitable silicone materials are disclosed in U.S. Patent Nos. 4,788,006; 4,341,799; 4,152,416; 3,964,500; 3,208,911; 4,364,837 and 4,465,619, all ofwhich are incorporated herein by reference.
Another suitable hydrophobic material which can be suspended in the composition has the formula
where i is a saturated or unsaturated, linear, branched or cyclic alkyl having 2 to 24 carbon atoms; M(+) is ^R^R+Rs; R2, R and R are hydrogen or a saturated or unsaturated, linear or branched alkyl or hydroxyalkyl having from 1 to 10 carbon atoms; and R5 is a saturated or unsaturated, linear, branched or cyclic alkyl or substituted alkyl having 2 to 24 carbon atoms. An example of such a material is lauramine oleate.
Other Adjuvants
Other suitable adjuvants for the composition include but are not limited to pH adjusters, emollients, conditioning agents, chelating agents, gelling agents, viscosifiers, colorants, fragrances, odor masking agents, UN stabilizer, preservatives, and any combination of any of the foregoing. Preferred pH adjusters include, but are not limited to, aminomethyl propanol, aminomethylpropane diol, triethanolamine, citric acid, sodium hydroxide, acetic acid, potassium hydroxide, lactic acid, and any combination of any of the foregoing.
Suitable conditioning agents include, but are not limited to, cyclomethicone, petrolatum, dimethicone, dimethiconol, silicone, quaternary amines and any combination of any of the foregoing.
The composition preferably contains less than about 0.5% by weight of preservatives, based upon 100% weight of total composition. More preferably, the composition contains from about 0.25 to about 0.5% by weight of preservatives, based upon 100%> weight of total composition.
The following examples are intended to describe the present invention without limitation. Table 1 contains three examples of moisturizing base gels made with phosphorylated starches. These are suitable for preparing surfactant free topical, oral, nasal, anal, opthalmic or vaginal compositions.
Table 1
Table 2 contains formulas of base gels that failed to support surfactant free formulas. These formulas separated overnight or soon after preparation, and therefore these gel bases are considered to be unsuitable for preparing surfactant free topical, oral, nasal, anal, opthahnic or vaginal compositions.
Table 2 - Base Composition
Table 3 contains a formula of a base gel that failed to support a surfactant free serum. This formula separated overnight or soon after preparation, and therefore this gel base is considered to be unsuitable for preparing surfactant free topical, oral, nasal, anal, opthahnic or vaginal compositions.
Table 3 - Serum
Table 4 contains a formula of a lotion spray that failed. This formula separated overnight or soon after preparation, and therefore this gel base is considered to be unsuitable for preparing surfactant free topical, oral, nasal, anal, opthahnic or vaginal compositions.
Table 4 - Lotion Spray
Table 5 contains a formula of a spray that failed. This formula separated overnight or soon after preparation, and therefore this gel base is considered to be unsuitable for preparing surfactant free topical, oral, nasal, anal, opthahnic or vaginal compositions.
Table 5 - Spray
Table 6 contains a formula of a surfactant-free cationic sunscreen cream that failed. This formula separated overnight or soon after preparation, and therefore this gel base is considered to be unsuitable for preparing surfactant free topical, oral, nasal, anal, opthahnic or vaginal compositions.
Table 6 - Surfactant-free Cationic Sunscreen Cream
Table 7 contains the gel base formulas used to make the products described in
Table 7 below.
Table 7 - Sclerotium / Carregeenum Base Variants
Table 8 contains a formula of a surfactant-free cationic sunscreen cream that failed. This formula separated overnight or soon after preparation, and therefore this gel base is considered to be unsuitable for preparing surfactant free topical, oral, nasal, anal, opthahnic or vaginal compositions.
Table 8 - Surfactant-free Cationic Sunscreen Cream
Examples 1-3
Preparation of Base Composition
Base compositions having the formulations in Table 9 below were prepared as follows. Structure Zea was sprinkled into deionized water. Germazide® MPB was added to the solution and mixed. Pemulen™ TR2 was added slowly to the solution while mixing. The solution was mixed until all the solids were dissolved and the solution was uniform. Triethanolamine was added and mixed until the solution was uniform and smooth. Merquat™ 550 was added to the solution and mixed to form the base composition.
Table 9
- Structure Zea is a hydroxypropyl distarch phosphate and is available from National Starch and Chemical Co. of Bridgewater, NJ.
2 - Germazide® MPB is a mixture of phenoxyethanol, chloφhenesin, glycerin, methylparaben, and benzoic acid and is available from Collaborative Laboratories, Inc. of East Setauket, NY.
3 - Pemulen™ TR2 is an acrylate/Cι.o-C3Q alkyl acrylate crosspolymer and is available from Goodrich Specialty Chemicals of Cleveland, OH.
- Carbopol 940 is carbomer and is available from Goodrich Specialty Chemicals of Cleveland, OH.
7 - Merquat™ 550 is polyquaternium-7 and available is from Calgon Coφoration of Pittsburgh, PA.
Three samples of the base composition were stored for 3 months at 4,25,40,50° C, respectively. After being stored overnight, 1 week, 2 weeks, 3 weeks, 1 month, 2 months and 3
months, each sample was observed and occasionally tested to deteirnine its pH and viscosity. The viscosity was measured with a Brookfield model LDNU+ viscometer (spindle E, 0.6 φm, 2 minutes, factor 7,812). The results are shown in Table 10 below.
Table 10
Prepare Moisturizing Cream for Oily Skin
A moisturizing cream for oily skin having the foπnulation of Table 11 below was prepared by mixing the ingredients with a propeller mixer until the composition was smooth and uniform.
Table 11
6 - The advanced moisture complex is a mixture of glycerine, water, sodium PCA, urea, trichalese, polyquaternium 51 and sodium hyaluronate.
7 - SanSurf® Cyclomethicone is a mixture of water, cyclopestasiloxane and phospholipids and is available from Collaborative Laboratories, hie. of East Setauket, NY.
8 -SanSurf® Polysynlane-30 is a mixture of water, hydrogenated polyisobutene, and phospholipids and is available from Collaborative Laboratories, Inc. of East Setauket, NY.
9 - Satin Finish is a mixture of water, phenyl trimethicone, cyclomethicone, dimethiconol, phospholipids, carbomer, and triethanolamine and is available from Collaborative Laboratories, Inc. of East Setauket, NY.
Three samples of the moisturizing cream were stored for two months at 25, 40, and 50° C, respectively. After being stored overnight, 1 week, 2 weeks, 3 weeks, 1 month, and 2 months, each sample was observed and occasionally tested to determine its pH and viscosity. The viscosity was measured with a Brookfield model LDNH+ viscometer (spindle E, 0.6 φm, 2 minutes, factor 7,812). The results are shown in Table 12 below.
Table 12
A sample of the moisturizing cream was also frozen and thawed three times. After each thawing, the cream was smooth and uniform.
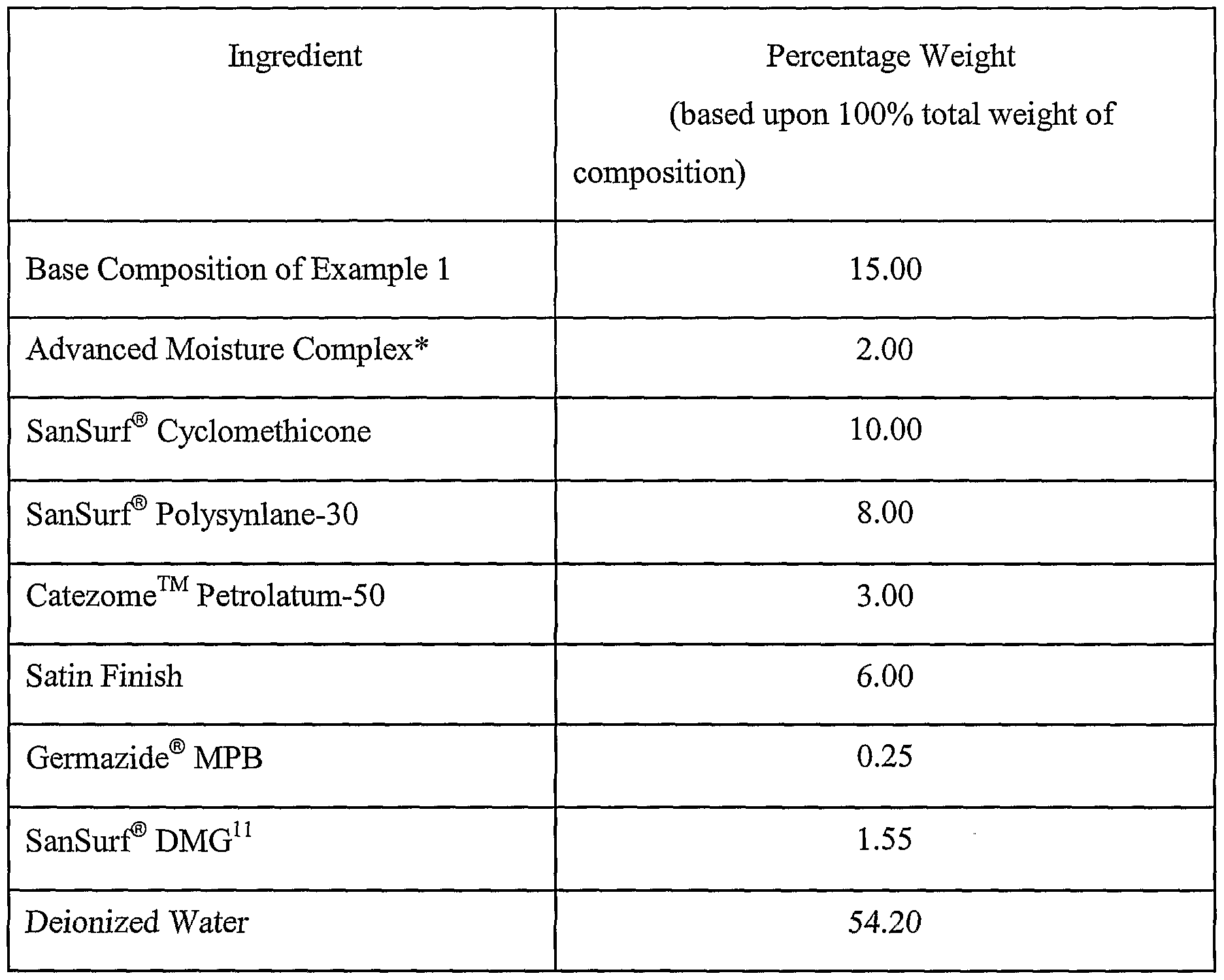
Example 5 Prepare Moisturizing Lotion for Oily Skin
A moisturizing lotion for oily skin having the formulation of Table 13 below was prepared by mixing the ingredients with a propeller mixer until the composition was smooth and uniform.
Table 13
* - The Advanced Moisture Complex is the same as in Example 4. 10 - Catezome™ Petrolatum-50 is a mixture of water, petrolatum, and stearamidopropyl dimethylamine stearate and is available from Collaborative Laboratories, Inc. of East Setauket,
NY.
Three samples of the moisturizing cream were stored for two months at 25, 40, and
50° C, respectively. After being stored overnight, 1 week, 2 weeks, 3 weeks, 1 month, and 2 months, each sample was observed and occasionally tested to detein ine its pH and viscosity. The
viscosity was measured with a Brookfield model LDNIJ+ viscometer (spindle E, 3 φm, 2 minutes, factor 1,562). The results are shown in Table 14 below.
Table 14
A sample of the moisturizing cream was also frozen and thawed three times. After each thawing, the cream was smooth and uniform.
Example 6 Prepare Moisturizing Serum for Normal Skin
A moisturizing serum for normal skin having the formulation of Table 15 below was prepared by first mixing the base composition and the deionized water and then mixing the other ingredients into the mixture with a propeller mixer until the mixture was homogeneous.
Table 15
* - The Advanced Moisture Complex is the same as in Example 4.
11 - SanSurf® DMG is a mixture of water, petrolatum, dimethicone, perfluoropolymethylisopropylether, stearamidopropyl dimethylamine, stearic acid, and tocopherol acetate, and is available from Collaborative Laboratories, Inc. of East Setauket, NY.
Three samples of the moisturizing serum were stored for two months at 25, 40, and 50° C, respectively. After being stored overnight, 1 week, 2 weeks, 3 weeks, 1 month, and 2 months, each sample was observed and occasionally tested to determine its pH and viscosity. The viscosity was measured with a Brookfield model LDNH+ viscometer (spindle 2, 1.5 φm, 1 minute, factor 200). The results are shown in Table 16 below.
Table 16
The viscosity and pH of the 40° C serum after 1 week and 4 weeks was 6.00 and 15,400 and 6.21 and 15,440, respectively. The viscosity andpH of the 50° C serum after 1 week and4 weeks was 6.26 and 15,840 and 6.38 and 15,520, respectively. The odor of the 25° C serum was observed at each time interval. The odor was characteristic of a moisturizing serum.
A sample of the moisturizing serum was also frozen and thawed three times. After each thawing, the serum was smooth and uniform.
Examples 7 and 8 Prepare Moisturizing Spray for Dry Skin
Moisturizing sprays for dry skin having the formulations of Table 17 below were prepared by mixing the ingredients with a propeller mixer until the composition was homogeneous.
Table 17
12 - Solarease
® is a mixture of ethylhexyl methoxycinnamate, butyl methoxydibenzoylmethane, cyclomethicone, phospholipids, and water and is available from Collaborative Laboratories, Inc. of East Setauket, NY.
13 - SanSurf® Gelled Polysynlane-30 Reg. is a mixture of water, hydrogenated polyisobutene, hydrogenated butylene/ethylene/styrene copolymer, hydrogenated etylene/propylene/styrene copolymer, and phospholipids and is available from Collaborative Laboratories, Inc. of East
Setauket, NY.
* - The Advanced Moisture Complex is the same as in Example 4.
A sample of the moisturizing spray of Example 8 was frozen and thawed three times. After each thawing, the spray was smooth and uniform.
Examples 9-12
The base compositions in Table 18 below were prepared as follows. The Structure Zea was slowly sifted into the deionized water and mixed until the Structure Zea was fully hydrated and all the solids were dissolved. The Stabylen-30™, Pemulin™ TR-2, Gerrnaben-IJ™, and triethanolamine were added sequentially and the mixture was mixed until uniform between additions.
These base compositions were found to be stable with acidic and basic dispersions and other ingredients.
Table 18
14 - Stabylen-30™ are acrylates/vinyl isodecanoate crosspolymers and is available from 3N Inc. of Weehawken, ΝJ.
15 - Germaben II™ is a mixture of propylene glycol, diazolidinyl urea, methylparaben, and propylparaben and is available from Sutton Laboratories of Chatham, ΝJ.
* - Example 11 was prepared with a 1% aqueous solution of Stabylen-30™ instead of a 2%o solution.
Examples 13-16
The base compositions in Table 19 below were prepared as follows. The Structure
Zea and Keltrol™ GFS were sprinkled into rapidly mixing deionized water until all the solids were dissolved and the solution was free offish eyes" (i.e. small clumps). The Stabylen-30™,
Germaben-π , and triethanolamine were added sequentially and the mixture was mixed until uniform between additions.
These base compositions were found to be stable with acidic and basic dispersions and other ingredients.
Table 19
16 - Keltrol™1 GFS is xantham gum and is available from Calgon Coφoration of Pittsburgh, PA. * - Example 13 was prepared with Keltrol™, which is xantham gum as well and is available from
Calgon Coφoration, instead of Keltrol™ GFS.
All patents, publications, applications, and test methods mentioned herein are hereby incoφorated by reference.
Many variations of the present invention will suggest themselves to those skilled in the art in light of the above, detailed description. All such obvious variations are within the full intended scope of the appended claims.