US20060159925A1 - High-intensity, persistent thermochromic compositions and objects, and methods for creating the same - Google Patents
High-intensity, persistent thermochromic compositions and objects, and methods for creating the same Download PDFInfo
- Publication number
- US20060159925A1 US20060159925A1 US11/311,289 US31128905A US2006159925A1 US 20060159925 A1 US20060159925 A1 US 20060159925A1 US 31128905 A US31128905 A US 31128905A US 2006159925 A1 US2006159925 A1 US 2006159925A1
- Authority
- US
- United States
- Prior art keywords
- thermochromic
- layer
- formulation
- preformed article
- materials
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- GACGWRPHOKBIIE-UHFFFAOYSA-N CCC(C)CC1=CC=C(OC(=O)C2=CC=C(C)C=C2)C=C1 Chemical compound CCC(C)CC1=CC=C(OC(=O)C2=CC=C(C)C=C2)C=C1 GACGWRPHOKBIIE-UHFFFAOYSA-N 0.000 description 1
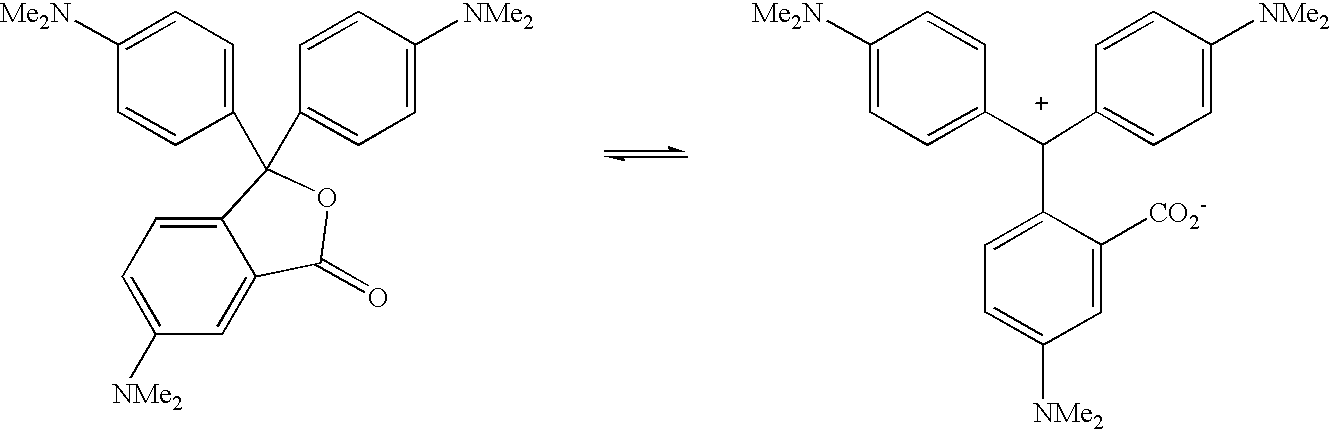
- YWVINFYPCWIGFT-UHFFFAOYSA-M CN(C)C1=CC=C(C(C2=CC=C(N(C)C)C=C2)C2=CC=C(N(C)C)C=C2C(=O)[O-])C=C1.CN(C)C1=CC=C(C2(C3=CC=C(N(C)C)C=C3)OC(=O)C3=CC(N(C)C)=CC=C32)C=C1 Chemical compound CN(C)C1=CC=C(C(C2=CC=C(N(C)C)C=C2)C2=CC=C(N(C)C)C=C2C(=O)[O-])C=C1.CN(C)C1=CC=C(C2(C3=CC=C(N(C)C)C=C3)OC(=O)C3=CC(N(C)C)=CC=C32)C=C1 YWVINFYPCWIGFT-UHFFFAOYSA-M 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/06—Luminescent, e.g. electroluminescent, chemiluminescent materials containing organic luminescent materials
-
- A—HUMAN NECESSITIES
- A63—SPORTS; GAMES; AMUSEMENTS
- A63B—APPARATUS FOR PHYSICAL TRAINING, GYMNASTICS, SWIMMING, CLIMBING, OR FENCING; BALL GAMES; TRAINING EQUIPMENT
- A63B37/00—Solid balls; Rigid hollow balls; Marbles
- A63B37/0003—Golf balls
- A63B37/0023—Covers
- A63B37/0024—Materials other than ionomers or polyurethane
-
- A—HUMAN NECESSITIES
- A63—SPORTS; GAMES; AMUSEMENTS
- A63B—APPARATUS FOR PHYSICAL TRAINING, GYMNASTICS, SWIMMING, CLIMBING, OR FENCING; BALL GAMES; TRAINING EQUIPMENT
- A63B37/00—Solid balls; Rigid hollow balls; Marbles
- A63B37/0003—Golf balls
- A63B37/005—Cores
- A63B37/0051—Materials other than polybutadienes; Constructional details
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B05—SPRAYING OR ATOMISING IN GENERAL; APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D—PROCESSES FOR APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D5/00—Processes for applying liquids or other fluent materials to surfaces to obtain special surface effects, finishes or structures
- B05D5/06—Processes for applying liquids or other fluent materials to surfaces to obtain special surface effects, finishes or structures to obtain multicolour or other optical effects
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B05—SPRAYING OR ATOMISING IN GENERAL; APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D—PROCESSES FOR APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D7/00—Processes, other than flocking, specially adapted for applying liquids or other fluent materials to particular surfaces or for applying particular liquids or other fluent materials
- B05D7/50—Multilayers
- B05D7/52—Two layers
- B05D7/54—No clear coat specified
- B05D7/542—No clear coat specified the two layers being cured or baked together
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B05—SPRAYING OR ATOMISING IN GENERAL; APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D—PROCESSES FOR APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D7/00—Processes, other than flocking, specially adapted for applying liquids or other fluent materials to particular surfaces or for applying particular liquids or other fluent materials
- B05D7/50—Multilayers
- B05D7/52—Two layers
- B05D7/54—No clear coat specified
- B05D7/546—No clear coat specified each layer being cured, at least partially, separately
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/02—Use of particular materials as binders, particle coatings or suspension media therefor
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K9/00—Tenebrescent materials, i.e. materials for which the range of wavelengths for energy absorption is changed as a result of excitation by some form of energy
- C09K9/02—Organic tenebrescent materials
-
- A—HUMAN NECESSITIES
- A63—SPORTS; GAMES; AMUSEMENTS
- A63B—APPARATUS FOR PHYSICAL TRAINING, GYMNASTICS, SWIMMING, CLIMBING, OR FENCING; BALL GAMES; TRAINING EQUIPMENT
- A63B2225/00—Miscellaneous features of sport apparatus, devices or equipment
- A63B2225/76—Miscellaneous features of sport apparatus, devices or equipment with means enabling use in the dark, other than powered illuminating means
-
- A—HUMAN NECESSITIES
- A63—SPORTS; GAMES; AMUSEMENTS
- A63B—APPARATUS FOR PHYSICAL TRAINING, GYMNASTICS, SWIMMING, CLIMBING, OR FENCING; BALL GAMES; TRAINING EQUIPMENT
- A63B37/00—Solid balls; Rigid hollow balls; Marbles
- A63B37/0003—Golf balls
-
- A—HUMAN NECESSITIES
- A63—SPORTS; GAMES; AMUSEMENTS
- A63B—APPARATUS FOR PHYSICAL TRAINING, GYMNASTICS, SWIMMING, CLIMBING, OR FENCING; BALL GAMES; TRAINING EQUIPMENT
- A63B43/00—Balls with special arrangements
- A63B43/06—Balls with special arrangements with illuminating devices ; with reflective surfaces
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1003—Carbocyclic compounds
- C09K2211/1007—Non-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1088—Heterocyclic compounds characterised by ligands containing oxygen as the only heteroatom
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/31504—Composite [nonstructural laminate]
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/31504—Composite [nonstructural laminate]
- Y10T428/31652—Of asbestos
- Y10T428/31663—As siloxane, silicone or silane
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/31504—Composite [nonstructural laminate]
- Y10T428/31786—Of polyester [e.g., alkyd, etc.]
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/31504—Composite [nonstructural laminate]
- Y10T428/31855—Of addition polymer from unsaturated monomers
- Y10T428/31935—Ester, halide or nitrile of addition polymer
Definitions
- thermochromic compositions comprising an effective amount of thermochromic materials formulated to yield change in color of high intensity.
- the present invention is also directed to thermochromic objects comprising at least one envirochromic layer, wherein said envirochromic layer comprises at least one thermochromic composition.
- Thermochromic objects may also additionally comprise at least one reflective layer, at least one colorant layer, and/or at least one protective layer.
- Such protective layers comprise photoluminescent fluorescent materials to retard photolytic degradation.
- the present invention is further directed to methods for creating thermochromic objects, said methods comprising the steps of obtaining a preformed article and applying thereto at least one envirochromic layer constructed by applying said thermochromic materials, and wherein one may additionally apply to said preformed article said reflective layer, and/or said colorant layer, and/or said protective layer.
- Desirable products are those that meet specific needs and/or provide added benefits to the consumer.
- These specific needs or added benefits may be safety controls or indicators, environmental information indicators, shelf life indicators, authentication and tamper indicators, fashion accessory benefits, and/or fun & entertainment benefits.
- These added benefits can be created, or indicators provided, by triggering a color change as a response to the product's environment.
- Envirochromic materials are those whose visible color changes due to emission, absorption reflection, or scattering of electromagnetic radiation. Such emission, absorption, reflection, or scattering of electromagnetic radiation results from a change in the material's environment. These changes, or “triggers,” include change in temperature, change in electromagnetic radiation, change in chemical environment, an electrical stimulus, etc.
- Color change can occur by changes in electromagnetic radiation, reflection, absorption or scattering.
- photochromism signifies color change triggered by electromagnetic radiation
- thermochromism signifies color change triggered by changes in temperature
- electrochromism signifies changes in color occurring due to gain or loss of electrons
- solvatochromism signifies color change resulting from changes in solvent polarity
- halochromism signifies color change caused by a change in pH
- ionochromism signifies color change caused by ions
- piezochromism signifies changes caused by mechanical pressure.
- Color change can also result from luminescent emissions.
- luminescent emissions we will use “luminescence” or “luminescent” to signify the color change.
- thermochromic temperature-triggered color changes as a consequence of changes in absorption reflection and/or scattering of electromagnetic radiation, hereinafter referred to as “thermochromic,” but also from luminescent emissions which will be designated as “luminescent” or “luminescence.”
- Thermochromism can be triggered, in general, by inorganic compounds, organic compounds, polymers, and sol-gels.
- thermochromic behavior arises from phase transition, change in ligand geometry, equilibria between different molecular structures, and change in the number of solvent molecules in the coordination sphere.
- Certain metal complex crystals comprising double salts of a transition metal such as cobalt, nickel, or manganese, and an aminic amide, such as hexamethylenetetramine, exhibit thermochromism. See, e.g., U.S. Pat. No. 4,717,710. These double salts discolor on releasing water when heated and resume the original color on absorption of moisture when cooled. Id.
- a transition metal such as cobalt, nickel, or manganese
- an aminic amide such as hexamethylenetetramine
- thermochromic temperature range is substantially from 50° C. to about 300° C. More specifically, the number of substances undergoing thermochromism at temperatures below 100° C. is limited to 2 or 3. For instance, in the case of Ag 2 HgI 4 , the thermochromism is from yellow to orange occurs at 50° C., and in the case of Cu 2 HgI 4 thermochromism from red to brown is brought about at 70° C. See, e.g., U.S. Pat. No. 4,028,118. Of course, the kind of color cannot be optionally chosen and the difference between colors before and after thermochromism is small. Moreover, since these metal complex crystals are not light-transmitting, it is not possible to use them as optical switches to hide/reveal an indicia, pattern or color in the layer below. Id.
- thermochromic materials These materials have heretofore been used as thermochromic materials but their applications are limited.
- thermochromism varies with molecular structure. It may be due to equilibrium between two molecular species, acid-base, keto-enol, lactim-lactam, or between stereoisomers or between crystal structures.
- Thermochromic liquid crystals show different colors at different temperature because of selective reflection of specific wavelength of electromagnetic radiation from their structure.
- these materials form a cholesteric liquid crystal.
- changes in temperature result in thermal expansion, which leads to a change in layer spacing and hence pitch, which results in a change in the wavelength of reflected light and hence a color change is observed with varying temperature.
- Thermochromic substances further include cholesteric liquid crystals and mixtures of cholesteric liquid crystals and nematic liquid crystals, but these substances also find greatly limited use because they are low in color density, have no selectivity in color and in color change temperature and are very expensive. See, e.g., U.S. Pat. No. 4,717,710.
- Liquid crystals generally exhibit thermochromism at temperatures ranging from ⁇ 10° C. to +200° C.
- the number of liquid crystals undergoing thermochromism at a temperature not exceeding 0° C. is very limited, namely 1 or 2.
- the color or thermochromism causing temperature cannot freely be chosen but is determined by the properties of the liquid crystals per se. These compounds are also chemically very sensitive; their properties are readily degraded upon contact with other substances.
- Thermochromic liquid crystal materials tend to be expensive and generally exhibit low color density. Hence, their use is not widespread, occurring in specialized situations
- Thermochromism arising from variations in stereoisomers in mostly associated with “overcrowded” ethylenes, such as Bianthrone, Dixanthylene, and Xanthylidenanthrone. These compounds are characterized by at least one ethylene group, a number of aromatic rings, and a hetero-atom, usually nitrogen or oxygen.
- the ethylene bond places a restriction on the molecular orientations possible, thereby increasing the energy barrier between different streoisomeric configurations.
- the temperature is increased, the molecule “switches” between different stereoisomers, this change being accompanied by a variation in color.
- thermochromism occurs at temperatures in excess of 150° C.
- bianthrone is colorless when solid, but forms green droplets above its melting point.
- the molecular rearrangement of an organic compound that arises from tautomerization can lead to an increase in the conjugation of the molecule, and consequently the formation of a new chromophore.
- Such molecular rearrangement can be effected by a change in temperature or by alteration of the polarity of the solvent and/or the pH of the system. Examples are, acid-base, keto-enol, and Lactim-Lactam equilibrium.
- thermochromic behavior Although fundamentally the chromism is pH-dependent, the temperature-dependence of the acid-base equilibrium means that pH sensitivity can result in thermochromic behavior. We present below one such example of crystal violet lactone.
- crystal violet Lactone does not exhibit thermochromism below a pH of 4. That is, even upon heating the ring opening does not occur and the colored form predominates. On the other hand, at higher pHs and upon heating equilibrium will shift to the left and the dye will become colorless.
- thermochromic system is one wherein molecular rearrangement occurs by a reaction between an electron donating compound referred to as a color former and an electron accepting compound referred to as a developer.
- compositions have the following advantages: (1) Thermochromic materials can be formulated for various colors; (2) The thermochromic materials gives a high color density; and (3) Depending on the kind of solvent, the color change temperature can be set over a wide range of low to high temperatures.
- thermochromic materials of this type generally comprise an electron donating compound, an electron accepting compound, and organic solvents to control the temperature and sensitivity of the thermochromic effect. It can be appreciated that since thermochromism is an equilibrium reaction, the two component systems can be very sensitive to their environment and hence are generally encapsulated to make them amenable to deployment in different formulations. See, e.g., U.S. Pat. Nos. 4,028,118; 4,421,560; 4,425,161; and 4,717,770.
- thermochromic materials which are generally very sensitive to their physical and chemical environment, which can comprise solvents, polymeric resin binders, stabilizing additives, pigments, dyes etc., which if not controlled, can quickly result in a degradation of the degree of color change, or even complete loss of thermochromic performance.
- thermochromic materials are encapsulated, to enable greater formulation flexibility for application to various substrates deploying a variety of techniques, the materials are still quite sensitive to their physical and chemical environment since the encapsulant is still porous.
- these materials are essential for the color change to be perceived as vibrant.
- thermochromic materials Even if the chemical environment does not impact the thermochromic materials, it can impact the capsule material. For example, the solvent can swell the polymeric capsule wall, thereby making it a scatterer of electromagnetic radiation. In cases where thermochromic materials are used as an optical switch, the changed color state will appear white and/or hazy instead of clear.
- thermochromic material environment is carefully selected so that the thermochromic performance is not degraded
- the solvents used can leach harmful materials from the substrate over which these materials are applied.
- thermochromic materials have generally been deployed as single layer applications, the color gamut is still fairly limited.
- pigments are added to colorants in order to expand the range of colors that can be achieved.
- color results from absorption of electromagnetic radiation creating colors with combinations of thermochromic materials of limited color range, and pigment colorants, is still quite restrictive in the range of bright colors that can be achieved.
- thermochromic materials are not only subject to photolytic degradation but by virtue of their sensitivity, need to be protected from mechanical forces such as abrasion, etc.
- thermochromic materials and mixtures of thermochromic materials will need to be adapted to provide the requisite color change.
- Thermochromic materials usually exist as solids or liquid crystals in a carrier medium.
- thermochromic materials When thermochromic materials are used as an optical switch, the theromochromic material needs to be formulated with clear resins and solvents that do not interact. Vibrant color layer formulations using solvents that do not leach are also desired.
- thermochromic objects also necessitates good adhesion to substrates and mechanical toughness such as scratch resistance, etc., specific requirements being dictated by the application.
- thermochromic materials and thermochromic objects created from such materials that can serve as safety indicators, fashion indicators, or create fun and entertainment by triggering temperature-activated color changes that are vibrant and impactful.
- the thermochromic objects can also be created in a variety of standard and emissive colors, also with high intensity.
- the thermochromic objects are also created to minimize photolytic degradation, do not degrade with moisture, and are mechanically robust, particularly in outdoor applications.
- thermochromic materials comprising an effective amount of thermochromic materials, at least one liquid carrier medium, at least one polymeric resin, and at least one formulation stabilizing additive, wherein said thermochromic materials are uniformly distributed within said formulation provide thermochromic formulations with high intensity and persistence.
- thermochromic formulation comprising an effective amount of thermochromic materials, at least one liquid carrier medium, at least one polymeric resin, and at least one formulation stabilizing additive, wherein said thermochromic materials are uniformly distributed within said formulation.
- the present invention is directed to a thermochromic formulation comprising an effective amount of thermochromic materials, at least one liquid carrier medium, at least one polymeric resin, and at least one formulation stabilizing additive, wherein said thermochromic materials are uniformly distributed within said formulation and wherein the theromchromic formulation has an FT of at least 90%.
- the present invention is directed to a thermochromic object comprising a performed article and at least one thermochromic layer, wherein the thermochromic layer results from the foregoing thermochromic formulations.
- the present invention is directed to a thermochromic object comprising a preformed article, at least one thermochromic layer, and at least one reflective layer, wherein the thermochromic layer results from the foregoing thermochromic formulations, wherein the reflective layer results from a reflective formulation, wherein the reflective layer is proximal to said preformed article, and wherein said thermochromic layer is distal to said preformed article.
- the present invention is directed to a thermochromic object comprising a preformed article, at least one thermochromic layer, at least one reflective layer, and at least one protective layer, wherein the thermochromic layer results from the foregoing thermochromic formulations, wherein the reflective layer results from a reflective formulation, wherein said protective layer results from a protective formulation, wherein the reflective layer is proximal to said preformed article, and wherein said protective layer is distal to said preformed article, and wherein said thermochromic layer is between said reflective layer and said protective layer.
- the present invention is directed to a thermochromic object comprising a preformed article, at least one thermochromic layer, and at least one protective layer, wherein the thermochromic layer results from the foregoing thermochromic formulations, wherein the protective layer results from a protective formulation, wherein the thermochromic layer is proximal to said preformed article, and wherein said protective layer is distal to said preformed article.
- the present invention is directed to a method for creating a thermochromic object, said method comprising the steps of obtaining a preformed article and applying to said preformed article at least one thermochromic layer, wherein said thermochromic layer results from the foregoing thermochromic formulations.
- the present invention is directed to a method for creating a thermochromic object, said method comprising the steps of obtaining a preformed article, applying to said preformed article at least one thermochromic layer, and applying to said preformed article at least one reflective layer, wherein the thermochromic layer results from the foregoing thermochromic formulations, wherein the reflective layer results from a reflective formulation, wherein the reflective layer is proximal to said preformed article, and wherein said thermochromic layer is distal to said preformed article.
- the present invention is directed to a method for creating a thermochromic object, said method comprising the steps of obtaining a preformed article, applying to said preformed article at least one thermochromic layer, applying to said preformed article at least one reflective layer, and applying to said preformed article at least one protective layer, wherein the thermochromic layer results from the foregoing thermochromic formulations, wherein the reflective layer results from a reflective formulation, wherein said protective layer results from a protective formulation, wherein the reflective layer is proximal to said preformed article, and wherein said protective layer is distal to said preformed article, and wherein said thermochromic layer is between said reflective layer and said protective layer.
- the present invention is directed to a method for creating a thermochromic object, said method comprising the steps of obtaining a preformed article, applying to said preformed article at least one thermochromic layer, and applying to said preformed article at least one protective layer, wherein the thermochromic layer results from the foregoing thermochromic formulations, wherein the protective layer results from a protective formulation, wherein the thermochromic layer is proximal to said preformed article, and wherein said protective layer is distal to said preformed article.
- FIG. 1 illustrates how absorption of ultraviolet radiation by a molecule excites it from a vibrational level in the electronic ground state to one of the many vibrational levels in the electronic excited state, such as singlet states and triplet states.
- FIG. 2 is a Jablonski Diagram illustrating processes that occur between the absorption and emission of electromagnetic radiation.
- FIG. 3 is a stylized depiction of an embodiment of the invention whereby a thermochromic object is created using a preformed article 1 with a thermochromic layer 2 applied thereto.
- FIG. 4 is a stylized depiction of an embodiment of the invention whereby a thermochromic object is created using a preformed article 1 with a reflective layer 3 and a thermochromic layer 2 applied thereto.
- FIG. 5 is a stylized depiction of an embodiment of the invention whereby a thermochromic object is created using a preformed article 1 with a theromchromic layer 2 and a protective layer 4 applied thereto.
- FIG. 6 is a stylized depiction of an embodiment of the invention whereby a thermochromic object is created using a preformed article 1 with a reflective layer 3 , a thermochromic layer 2 , and a protective layer 4 applied thereto.
- FIG. 7 is a stylized depiction of an embodiment of the invention whereby transfer technology is used to obtain the thermochromic object.
- FIG. 8 is a stylized depiction of an embodiment of the invention whereby a thermochromic object is created using a preformed article 1 with a reflective layer 3 , a first thermochromic layer 2 , a second thermochromic layer 5 , a first protective layer 4 , and second protective layer 6 applied thereto.
- FIG. 9 is a stylized depiction of an embodiment of the invention whereby a thermochromic object is created using a preformed article 1 with a reflective layer 3 , a first thermochromic layer 2 , a second thermochromic layer 5 , and a first protective layer 4 applied thereto.
- FIG. 10 is a stylized depiction of an embodiment of the invention whereby a thermochromic object is created using a preformed article 1 with a reflective layer 3 , a first thermochromic layer 2 , a first protective layer 4 , and second protective layer 6 applied thereto.
- thermochromic formulations As noted above, the present invention generally relates to thermochromic formulations, thermochromic objects comprising preformed articles onto which said thermochromic formulations have been applied, and to methods for creating said thermochromic objects.
- Luminescence is defined as the emission of electromagnetic radiation from any substance. Luminescence occurs from electronically excited states. As seen in FIG. 1 , absorption of ultraviolet radiation by a molecule excites it from a vibrational level in the electronic ground state to one of the many vibrational levels in the electronic excited states. The electronic states of most organic molecules can be divided into singlet states and triplet states.
- plasma state refers to when all electrons in the molecule are spin-paired.
- triplet state refers to when one set of electron spins is unpaired.
- the excited state is usually the first excited state. A molecule in a high vibrational level of the excited state will quickly fall to the lowest vibrational level of this state by losing energy to other molecules through collision. The molecule will also partition the excess energy to other possible modes of vibration and rotation.
- Luminescent materials are those which emit electromagnetic radiation. Characterizing luminescent materials requires consideration of: (1) the excitation source, (2) the nature of the emission, and (3) whether or not additional stimulation is required to cause emission.
- luminescent materials excited by electromagnetic radiation are referred to herein as “photoluminescent.”
- Luminescent materials excited by electrical energy are referred to herein as “electroluminescent.”
- Luminescent materials excited by a chemical reaction are referred to herein as “chemiluminescent.”
- this may be either fluorescence or phosphorescence.
- a “fluorescent” material stores electromagnetic radiation and releases it rapidly, in about 10 ⁇ 12 seconds or less.
- a “phosphorescent” material stores electromagnetic radiation and releases it gradually, in about 10 ⁇ 8 seconds or more.
- FIG. 2 Processes that occur between the absorption and emission of electromagnetic radiation are usually illustrated using a Jablonski Diagram, such as the one found in FIG. 2 .
- Ground, first, and second electronic states are depicted in FIG. 2 by S 0 , S 1 , and S 2 , respectively.
- the fluorophores can exist in a number of vibrational energy levels, denoted by 0, 1, 2, etc. Transitions between states are depicted in FIG. 2 as vertical lines to illustrate the instantaneous nature of electromagnetic radiation absorption.
- Fluorescence occurs when a molecule returns, by emission of a photon, from the excited singlet state to the electronic ground state. If the photon emission occurs between states of the same spin state, that is, from S 1 to S 0 , it is characterized as fluorescence.
- Phosphorescence occurs when a molecule goes from the ground state to a metastable state such as T1, a triplet state, and then the metastable state slowly decays back to the ground state S 0 , via photon emission. Hence, if the spin states between initial and final energy levels are different, that is emission occurs between T 1 to S 0 , it is characterized as phosphorescence.
- thermochromic is defined as characterizing a change in absorption, reflection, and/or scattering of electromagnetic radiation with a change in temperature to cause a change in the perceived color, wherein the color change may be from colorless to colored, or colored to colorless, or from one color to another.
- Thermochromic materials are defined to mean materials that undergo a change in absorption, reflection, and/or scattering of electromagnetic radiation with a change in temperature to cause a change in the perceived color of the materials wherein the color change may be from colorless to colored, or colored to colorless, or from one color to another.
- Thermochromic objects are defined to mean objects that undergo a change in absorption, reflection, and/or scattering with a change in temperature to cause a change in the perceived color of the object wherein the color change may be from colorless to colored, or colored to colorless or from one color to another.
- “Negative thermochromism” is defined to describe a temperature-triggered color change resulting from a change in absorption, reflection, and/or scattering wherein the color change is from a colored state to a colorless state.
- Pelsitive thermochromism is defined to describe a temperature-triggered color change resulting from a change in absorption, reflection, and/or scattering of electromagnetic radiation wherein the color change is from a colorless to colored state.
- Nemism is defined to describe a temperature-triggered color change resulting from a change in absorption, reflection, and/or scattering of electromagnetic radiation wherein the color change is from one color to another color.
- Liquid carrier medium is a liquid that acts as a carrier for materials distributed in a solid state and/or dissolved therein.
- a “formulation” is a liquid carrier medium, as defined above, comprising at least one material either dissolved and/or distributed in a solid state within said liquid carrier medium.
- a “dispersion” is a formulation, as defined above, wherein said material is a solid distributed in the liquid carrier medium.
- thermochromic formulation is a formulation, as defined above, which additionally comprises thermochromic materials as defined above.
- thermochromic layer is a film resulting from at least one thermochromic formulation that is substantially dry, as characterized by the residual liquid carrier medium being in the range of 1-5 weight % of the total weight of the film.
- a “reflective formulation” is a formulation, as defined above, which comprises at least a polymeric resin in a liquid carrier medium, as defined above, and further comprises at least one colorant (white or non-white).
- a “reflective layer” is a film resulting from at least one reflective formulation that is substantially dry, as characterized by the residual liquid carrier medium being in the range of 1-5 weight % of the total weight of the film.
- a “white reflectance layer” is one that reflects 95% of visible electromagnetic radiation incident upon it.
- a “protective formulation” is a formulation, as defined above, which comprises at least a polymeric resin selected for environmental or mechanical protection of the underlying article, upon application onto said article.
- a “protective layer” is a film resulting from at least one protective formulation that is substantially dry, as characterized by the residual liquid carrier medium being in the range of 1-5 weight % of the total weight of the film.
- a “stabilizing additive” is a material added to a formulation comprising solid particles or a dispersion to uniformly distribute, prevent agglomeration, and/or prevent settling of solid materials in said dispersion in said liquid carrier medium to result in an enhancement of luminous intensity.
- Such stabilizing additives generally comprise dispersants and/or rheology modifiers.
- a “preformed article” is any article onto which at least one layer may be applied.
- a preformed article may be rigid or flexible.
- visible electromagnetic radiation is characterized by electromagnetic radiation with wavelengths in the region of 400 nanometers (“nm”) to 700 nm.
- FT Frm Transmissivity
- Photolytic degradation is deterioration, degradation, or change in properties, such as observed color, that is initiated by electromagnetic radiation.
- thermochromic materials that are formulated to be liquid mixtures with stabilizing additives comprising polymeric resin binders, dispersants, rheology modifiers, and wetting agents so as to be highly transmissive of visible electromagnetic radiation in the colorless thermochromic state upon application to any article.
- the present invention also relates to the use of photoluminescent fluorescent pigment materials to render color or aid in rendering color upon triggering of thermochromic materials into a colorless state.
- the present invention also relates to the use of the luminescent fluorescent dye materials such that they exist as solid solutions in the polymeric resin matrix, thereby creating high ratio of emission per unit weight of material deployed for rendering color or aid in rendering color upon triggering of the thermochromic materials into a colorless state
- the present invention also relates to the use of photostabilizers either singly or in combination or with a combined functionality in a single molecule to retard the photolytic degradation of said thermochromic and photoluminescent fluorescent materials cited above.
- thermochromic objects comprising at least one thermochromic layer, wherein said thermochromic layer is highly transmissive of visible electromagnetic radiation and wherein the thermochromic materials have been triggered into a colorless state.
- thermochromic objects comprising at least one thermochromic layer, as defined above, but which may additionally comprise photoluminescent fluorescent pigments, dyes, or both, as described above.
- thermochromic objects comprising at least one thermochromic layer, as defined abov,e but which may additionally comprises photostabilizers, as described above
- thermochromic object which comprises a thermochromic layer, as defined above, wherein the thermochromic layer functions as an optical switch to transition from either a colored state to a colorless state or from a colorless state to a colored state.
- thermochromic object which comprises a reflective layer comprising polymeric resin and white or colored pigments, wherein the reflective layer is highly reflective of unabsorbed visible electromagnetic radiation and wherein such reflective layer is deployed in conjunction with a thermochromic layer.
- thermochromic objects comprising a reflective layer, as defined above, and additionally comprising photoluminescent fluorescent pigments wherein the reflective layer is highly reflective of unabsorbed visible electromagnetic radiation.
- thermochromic object comprising a reflective layer, as defined abov,e and additionally comprising photoluminescent fluorescent dyes wherein the photoluminescent fluorescent dye exists as a solid solution in polymeric matrix wherein the reflective layer is highly reflective of unabsorbed visible electromagnetic radiation.
- thermochromicobject comprising a reflective layer as defined above which additionally comprises photostabilizers as defined above to retard the photolytic degradation of said thermochromic and photoluminescent fluorescent materials cited above
- thermochromic object comprises at least one reflective layer and at least one thermochromic layer
- the reflective layer is applied first, so that it is proximal to the preformed article.
- the thermochromic layer is applied after the reflective layer, so that said thermochromic layer is distal to said preformed article. If transfer technology is used, it must be used such that the result is the same.
- thermochromic object comprising at least one protective layer which comprises a polymeric resin and wherein the protective layer is deployed in conjunction with an thermochromic layer and wherein the protective layer provides physical protection to the thermochromic layer.
- thermochromic object comprising at least one protective layer as defined above but which additionally comprises photostabilizers as described above.
- thermochromic object comprises at least one thermochromic layer and at least one protective layer
- the thermochromic layer is applied first, so that it is proximal to said preformed article.
- the protective layer is applied after the thermochromic layer, so that said protective layer is distal to said preformed article. If transfer technology is used, it must be used such that the result is the same.
- thermochromic object comprises at least one reflective layer, at least one thermochromic layer, and at least one protective layer
- the reflective layer is applied first, so that it is proximal to said preformed article.
- the thermochromic layer is applied next and then the protective layer is applied. Therefore, the reflective layer is proximal to the preformed article, the protective layer is distal to said preformed article, and said thermochromic layer is between said reflective layer and said protective layer. If transfer technology is used, it must be used such that the result is the same.
- the present invention also relates to the method of making a thermochromic object with at least one thermochromic layer.
- the present invention also relates to the method of making a thermochromic object with at least one reflective layer and one thermochromic layer.
- the present invention also relates to the method of making a thermochromic object with at least one thermochromic layer and at least one protective layer.
- the present invention also relates to the method of making a thermochromic object with at least one colorant layer and at least one thermochromic layer.
- the present invention relates to the method of making a thermochromic object with at least one reflective layer, at least one thermochromic layer and at least one protective layer.
- the present invention relates to the method of making a thermochromic object with at least one colorant layer, at least one thermochromic layer and at least one protective layer.
- the present invention relates to the method of making a thermochromic object with at least one reflective layer, at least one colorant layer, at least one thermochromic layer and at least one protective layer.
- New products have surprisingly been developed that address the problems associated with various compounds and their uses, including developing products which have color changing indicators based on thermochromic, fluroscent photochromic, phosphorescent photochromic compounds or a combination thereof and that are constructed from a variey of substrate materials such as PVC acrylics, urethanes polyester, nylon, etc.
- the substrate or surface construction materials or components may be rigid or flexible.
- a system construction has also been developed such that the visual impact of the color change is striking, the indicator indicia are robust, the applied images patterns or indicia are photolytically stable and the product is not only durable for the user but also environmentally stable.
- These new products are also desirable and useful to consumers in many fields of use, including food products, entertainment, sports, transportation, weather protection, decorating, indoor and outdoor sanitation or a combination thereof.
- a multilayer system construction or layered material has been designed so as to create a visually striking impact of the color change, to be durable with respect to scratches and abrasions and to have weatherometric robustness, such as photolytic stability and atmospheric stability for the indicator indicia or images.
- One contemplated embodiment comprises a three functional layer structure with a base layer providing functionality for maximizing or that maximizes the desired visual impact of the color change of at least part of the layered material, a thermochromic layer providing functionality for triggering a state change that is, either colorless to colored, or colored to colorless, or from one color to another, and a protective layer that provides functionality for handling and weatherometric robustness for the underlying image.
- the protective layer can also provide a visual enhancement function when desired by providing a reflective component or gloss for image viewing.
- the base functional layer may produce the desired functionality either by a single or multiple layers of material, depending on the application and product.
- the base layer may be constructed either by blending the final color dye or pigment before fabrication of the layered material, such as by extrusion or molding or formation of plastic, glass, paper etc., or by a coating application process such as gravure, flexo, roll or blade coating etc., or by printing application processes such as screen printing or pad printing etc.
- a base layer construction would to apply a layer containing a dye or pigment material including one or more fluorescent dyes or pigments that will render a brilliant final color.
- the thermochromic layer (applied on top of this layer) would generally function as an optical switch going from colored to colorless.
- thermochromic layer having the appropriate dye or pigment for the desired visual impact.
- Another application of a base layer construction is where the base layer contains just a white reflective pigment coating to maximze the color rendering of the thermochromic materials. In this type of application the thermochromic layer would go from either colorless to colored or from one color to another.
- the base layer may also comprise multiple layers.
- An example of a two layer base functional layer is contemplated when the base layer is being applied to a rigid or flexible plastic or other material containing a strong absorbing color different from the final color.
- the base layer will consist of a hidden layer consisting of a white reflective pigment application followed by the layer rendering the final color such as that described above.
- the base functional layer could become a three layer construction. In this case, one has to first create a desired oversized (oversized relative to final color image) contrast color image followed by a white reflective image followed by final color image application.
- the base layer in addition to serving the function of enhancing the visual impact of the final color, will also serve as a platform for the additional layers and/or functional layers. Therefore, it is important to consider the components of the base layer with respect to the components of additional layers in order to avoid color bleeding, undesirable chemical reactions between layers of materials and deterioration of the desired visual impact of the layered materials.
- the second layer applied on top of the base layer will contain the thermochromic layer.
- the thermochromic layer may have one or more layers, where the layer or layers comprise at least one layer of envirochromic dyes, pigments or inks.
- Envirochromic dyes, pigments or inks are those that change color or texture with a changing environment, such as heat, cold, rain, sunshine, UV rays, snow, dark, light or a combination thereof.
- Envirochromic dyes, pigments or inks are contemplated to be those described herein earlier, conventional envirochromic dyes, pigments or inks, envirochromic dyes, pigments or inks that are yet to be developed or a combination thereof.
- Envirochromic inks, dyes and pigments may be clear until triggered, opaque until triggered or a particular color until triggered. Once triggered by an environmental condition, the ink, dye or pigment will change—whether it changes from clear to opaque or colored, from opaque to clear or colored or from colored to opaque or clear.
- Dispersing agents are surface-active substances of anionic, cationic, or neutral structure. These substances are added in a small amount, either directly to the solid or to the dispersion medium. Furthermore, it is known that even after complete deflocculation of the solid agglomerates into primary particles, re-agglomeration occurs after the dispersion process. In such a case, the effort expended to produce a dispersion is partially or completely negated.
- amino- and amide-functional poly- and oligocopolymers based on polyamines and polycaprolactones are used for the dispersion of magnetic pigments.
- European Patent No. 0 713 894 describes the use of amino-functional polylactones for coatings and printing inks.
- amine-functional polyacrylates such as those disclosed in European Patent No. 0 311 157 and in U.S. Pat. No. 3,980,602 are used for the stabilisation of organic and inorganic pigments.
- Amine-functional polymers based on polyisocyanates constitute a further group. See, e.g., European Patent Nos. 0 159 678 and 0 438 836.
- Suitable dispersing aids that minimize agglomeration require very low levels of energy are cited for example an acrylic acid-acrylamide polymer such as those cited in U.S. Pat. No. 6,596,816, incorporated herein by reference for all purposes, or salts of an amine functional compound and an acid such as those cited in U.S. Pat. No. 6,111,054, also incorporated herein by reference for all purposes.
- “Rheology Modifiers” are those substances which generally can build viscosity in liquid dispersion formulations, thereby retarding settling of pigment materials while at the same time significantly lowering viscosity upon application of shear, to enhance smooth applicability of such formulations onto articles.
- materials such as colloidal silica or fumed silica and magnesium aluminum silicate clays, such as bentonite, not only as thixotropic modifiers to prevent sagging and running of luminescent formulation as it is applied to the object but also as suspending fillers, that is, for minimizing settling of dense pigment particles such as phosphor particles. See, e.g., U.S. Pat. No. 6,207,077.
- thermochromic layer The bigger disadvantage of their use in luminescent coatings such as the thermochromic layer is that they lead to turbidities and haze rather than transparent coatings. Moreover, handling dry pulverulent products which give rise to dusts in the course of processing is undesirable.
- the present invention employs polymeric urea-urethanes in aprotic polar solvents as rheology modifiers.
- This class of rheology modifiers can be used satisfactorily, that is without any scattering of electromagnetic radiation, and without any significant build up of viscosity. Thus, this class serves not only as rheology modifiers but also to minimize settling of the dense pigment particles. Examples of such urea-urethanes can be found, for example, in U.S. Pat. No. 6,617,468 and U.S. Pat. No. 6,870,024, incorporated herein by reference for all purposes.
- the resulting surface may not be smooth. Instead, the surface may be less structured, referred to as having a wavy surface or as having an orange peel-like surface. These surfaces may be finely structured, with a short wave, or coarsely structured, with a long wave.
- the structure depends on the nature and composition of the coating compositions; for example, on whether these coating compositions comprise solvents or else are solvent-free, as in the case of powder coating materials.
- these coating compositions comprise solvents or else are solvent-free, as in the case of powder coating materials.
- powder coating materials it is absolutely necessary to add leveling agents, since without these leveling agents it is impossible to achieve a surface which is in any way smooth.
- poly(meth)acrylates and polysiloxanes which may be used as leveling promoters for coatings.
- the compounds generally comprise polydimethylsiloxanes, polymethylalkylsiloxanes, or else polyether- or polyester-modified polydimethyl- or polymethylalkylsiloxanes.
- the products used possess in some cases molecular weights of up to 100,000.
- the action of all these products is based on surface activity at the liquid/gas interface: owing to a certain incompatibility with the actual binder of the coating system, these products adopt an orientation to the interface. This incompatibility may be increased by raising the molecular weight of these polymers.
- a disadvantage then, however, is that owing to this incompatibility there can be cases wherein the scattering of electromagnetic radiation or haze of the layer becomes high, thereby resulting in significant reduction in luminous intensity.
- the present invention employs branched polymers comprising a free-radically or ionically polymerized base molecule into which monoethylenically unsaturated macromonomeric units have been incorporated by copolymerization. Examples of such polymers may be found in U.S. Pat. No. 6,710,127, incorporated herein by reference for all purposes.
- Deaerators are those substances which minimize entrained air.
- Defoamers are those substances that allow easier dissipation of entrained air.
- a significant amount of air entrainment can cause scattering of electromagnetic radiation and, hence, a reduction in luminous intensity.
- thermochromic pigment in a binder of nitrocellulose and diluted with 2-methoxyethanol. This gave pigment solids of 40% of dry pigment to 60% Nitrocellulose binder. The solids were contained in a solvent mixture at 45% solids. This material was coated over plasticized PVC to give a dry coating thickness of 18 microns using a screen printing process.
- the color of the thermochromic pigment at room temperature was selected so that it contrasted the color of the PVC.
- the opacity of the thermochromic layer at room temperature masked the PVC layer below it. The thermochromic layer became translucent when heated through normal body contact to reveal the contrasting base color of the PVC underneath.
- thermochromic pigment in 48% Nitrocellulose binder.
- the pigment and binder were prepared in a solvent mixture containing Toluene, Hexane, and Hydrocarbon solvents at a solids content of 40%. This material was applied using a silk screen to give a dry coating thickness of 22 microns.
- a yellow fluorescent pigment was dispersed in 2-methoxyethanol at 50% solids using the wetting agent TegoWet 550. This dispersion was added to an acrylic binder (NeoCryl B-735) to give solids of 20% in 2-methoxyethanol.
- the ink was then applied to plasticized PVC using a screen print to achieve a 15 micron thickness, which provided for an image that contrasted the base color of the PVC. Over this was applied the formulation from Example 2 at a thickness of 26 microns.
- the color of the thermochromic layer at room temperature contrasted with the fluorescent layer below it. In this case, the thermochromic layer was dark blue compared to the fluorescent yellow of the layer below the thermochromic layer.
- the opacity of the thermochromic layer masked the fluorescent layer below it. The image was such that when warmed, the thermochromic pigment became translucent and revealed the fluorescent layer printed underneath.
- Thermochromic pigment was mixed with 10% 2-methoxyethanol and then dispersed in plastisol to give a pigment concentration of 40% in the final mixture. This was then applied over a polyester fabric using a applicator to give a 1 mil ending thickness.
Abstract
Disclosed are thermochromic formulations, comprising an effective amount of thermochromic materials, which exhibit high luminous intensity and persistence. Also disclosed are thermochromic objects formed by applying at least one thermochromic layer, formed from thermochromic formulations, to preformed articles. Further disclosed are methods for creating thermochromic objects.
Description
- This application claims priority to U.S. Provisional Patent Application Ser. No. 60/637,535, filed Dec. 20, 2004 (Attorney Docket No. 7044531001), titled, “Layered Envirochromic Materials, Applications and Methods of Preparation Thereof,” which is incorporated by reference herein for all purposes.
- 1. Field of the Invention
- The present invention is directed to thermochromic compositions comprising an effective amount of thermochromic materials formulated to yield change in color of high intensity. The present invention is also directed to thermochromic objects comprising at least one envirochromic layer, wherein said envirochromic layer comprises at least one thermochromic composition. Thermochromic objects may also additionally comprise at least one reflective layer, at least one colorant layer, and/or at least one protective layer. Such protective layers comprise photoluminescent fluorescent materials to retard photolytic degradation. The present invention is further directed to methods for creating thermochromic objects, said methods comprising the steps of obtaining a preformed article and applying thereto at least one envirochromic layer constructed by applying said thermochromic materials, and wherein one may additionally apply to said preformed article said reflective layer, and/or said colorant layer, and/or said protective layer.
- 2. Description of the Related Art
- Modern consumers are looking for added information and features from the products that they purchase. Desirable products are those that meet specific needs and/or provide added benefits to the consumer. These specific needs or added benefits may be safety controls or indicators, environmental information indicators, shelf life indicators, authentication and tamper indicators, fashion accessory benefits, and/or fun & entertainment benefits. These added benefits can be created, or indicators provided, by triggering a color change as a response to the product's environment.
- Envirochromic materials are those whose visible color changes due to emission, absorption reflection, or scattering of electromagnetic radiation. Such emission, absorption, reflection, or scattering of electromagnetic radiation results from a change in the material's environment. These changes, or “triggers,” include change in temperature, change in electromagnetic radiation, change in chemical environment, an electrical stimulus, etc.
- Color change can occur by changes in electromagnetic radiation, reflection, absorption or scattering. Thus, for example, photochromism signifies color change triggered by electromagnetic radiation; thermochromism signifies color change triggered by changes in temperature; electrochromism signifies changes in color occurring due to gain or loss of electrons; solvatochromism signifies color change resulting from changes in solvent polarity; halochromism signifies color change caused by a change in pH; ionochromism signifies color change caused by ions; tribochromism caused by changes in mechanical friction; and piezochromism signifies changes caused by mechanical pressure.
- Color change can also result from luminescent emissions. Hence, when dealing with color changes resulting from emissions, we will use “luminescence” or “luminescent” to signify the color change.
- This invention deals with color changes not only resulting from temperature-triggered color changes as a consequence of changes in absorption reflection and/or scattering of electromagnetic radiation, hereinafter referred to as “thermochromic,” but also from luminescent emissions which will be designated as “luminescent” or “luminescence.”
- Thermochromism can be triggered, in general, by inorganic compounds, organic compounds, polymers, and sol-gels.
- Inorganic Compound Thermochromism
- Many metals and inorganic compounds are known to exhibit thermochromic behavior either as solids or in solution. It has been suggested that such thermochromic behavior arises from phase transition, change in ligand geometry, equilibria between different molecular structures, and change in the number of solvent molecules in the coordination sphere.
- Certain metal complex crystals comprising double salts of a transition metal such as cobalt, nickel, or manganese, and an aminic amide, such as hexamethylenetetramine, exhibit thermochromism. See, e.g., U.S. Pat. No. 4,717,710. These double salts discolor on releasing water when heated and resume the original color on absorption of moisture when cooled. Id.
- However, in these metal complex crystals, the thermochromic temperature range is substantially from 50° C. to about 300° C. More specifically, the number of substances undergoing thermochromism at temperatures below 100° C. is limited to 2 or 3. For instance, in the case of Ag2 HgI4, the thermochromism is from yellow to orange occurs at 50° C., and in the case of Cu2 HgI4 thermochromism from red to brown is brought about at 70° C. See, e.g., U.S. Pat. No. 4,028,118. Of course, the kind of color cannot be optionally chosen and the difference between colors before and after thermochromism is small. Moreover, since these metal complex crystals are not light-transmitting, it is not possible to use them as optical switches to hide/reveal an indicia, pattern or color in the layer below. Id.
- These materials have heretofore been used as thermochromic materials but their applications are limited.
- Organic Compound Thermochromism
- The mechanism responsible for thermochromism varies with molecular structure. It may be due to equilibrium between two molecular species, acid-base, keto-enol, lactim-lactam, or between stereoisomers or between crystal structures.
- Thermochromic liquid crystals show different colors at different temperature because of selective reflection of specific wavelength of electromagnetic radiation from their structure. In an appropriate temperature range intermediate between a low-temperature crystalline phase and a high-temperature isotropic liquid phase, these materials form a cholesteric liquid crystal. In a cholesteric liquid crystal, changes in temperature result in thermal expansion, which leads to a change in layer spacing and hence pitch, which results in a change in the wavelength of reflected light and hence a color change is observed with varying temperature. We present below an example of such a material that is used in the manufacture of a thermochromic printing ink.
- Thermochromic substances further include cholesteric liquid crystals and mixtures of cholesteric liquid crystals and nematic liquid crystals, but these substances also find greatly limited use because they are low in color density, have no selectivity in color and in color change temperature and are very expensive. See, e.g., U.S. Pat. No. 4,717,710.
- Liquid crystals generally exhibit thermochromism at temperatures ranging from −10° C. to +200° C. However, the number of liquid crystals undergoing thermochromism at a temperature not exceeding 0° C. is very limited, namely 1 or 2. The color or thermochromism causing temperature cannot freely be chosen but is determined by the properties of the liquid crystals per se. These compounds are also chemically very sensitive; their properties are readily degraded upon contact with other substances.
- Thermochromic liquid crystal materials tend to be expensive and generally exhibit low color density. Hence, their use is not widespread, occurring in specialized situations
- Thermochromism arising from variations in stereoisomers in mostly associated with “overcrowded” ethylenes, such as Bianthrone, Dixanthylene, and Xanthylidenanthrone. These compounds are characterized by at least one ethylene group, a number of aromatic rings, and a hetero-atom, usually nitrogen or oxygen. The ethylene bond places a restriction on the molecular orientations possible, thereby increasing the energy barrier between different streoisomeric configurations. As the temperature is increased, the molecule “switches” between different stereoisomers, this change being accompanied by a variation in color. For a majority of compounds that exhibit this behavior, thermochromism occurs at temperatures in excess of 150° C. For example, bianthrone is colorless when solid, but forms green droplets above its melting point.
- The molecular rearrangement of an organic compound that arises from tautomerization can lead to an increase in the conjugation of the molecule, and consequently the formation of a new chromophore. Such molecular rearrangement can be effected by a change in temperature or by alteration of the polarity of the solvent and/or the pH of the system. Examples are, acid-base, keto-enol, and Lactim-Lactam equilibrium.
-
- It should be noted that crystal violet Lactone does not exhibit thermochromism below a pH of 4. That is, even upon heating the ring opening does not occur and the colored form predominates. On the other hand, at higher pHs and upon heating equilibrium will shift to the left and the dye will become colorless.
- The mechanism described above has been commercialized the most, and there is a wide variety of thermochromic materials available. Specifically, the commonly-employed thermochromic system is one wherein molecular rearrangement occurs by a reaction between an electron donating compound referred to as a color former and an electron accepting compound referred to as a developer.
- These compositions have the following advantages: (1) Thermochromic materials can be formulated for various colors; (2) The thermochromic materials gives a high color density; and (3) Depending on the kind of solvent, the color change temperature can be set over a wide range of low to high temperatures.
- To summarize, thermochromic materials of this type generally comprise an electron donating compound, an electron accepting compound, and organic solvents to control the temperature and sensitivity of the thermochromic effect. It can be appreciated that since thermochromism is an equilibrium reaction, the two component systems can be very sensitive to their environment and hence are generally encapsulated to make them amenable to deployment in different formulations. See, e.g., U.S. Pat. Nos. 4,028,118; 4,421,560; 4,425,161; and 4,717,770.
- It can be seen that there are a wide variety of thermochromic materials but which are generally very sensitive to their physical and chemical environment, which can comprise solvents, polymeric resin binders, stabilizing additives, pigments, dyes etc., which if not controlled, can quickly result in a degradation of the degree of color change, or even complete loss of thermochromic performance. Even for the case when thermochromic materials are encapsulated, to enable greater formulation flexibility for application to various substrates deploying a variety of techniques, the materials are still quite sensitive to their physical and chemical environment since the encapsulant is still porous. For the use of these materials as envirochromic materials that serve as indicators, as described above, it is essential for the color change to be perceived as vibrant.
- Even if the chemical environment does not impact the thermochromic materials, it can impact the capsule material. For example, the solvent can swell the polymeric capsule wall, thereby making it a scatterer of electromagnetic radiation. In cases where thermochromic materials are used as an optical switch, the changed color state will appear white and/or hazy instead of clear.
- For single layer constructions, even if the thermochromic material environment is carefully selected so that the thermochromic performance is not degraded, the solvents used can leach harmful materials from the substrate over which these materials are applied.
- Even though progress has been made over the years in expanding the colors and temperature ranges available, since thermochromic materials have generally been deployed as single layer applications, the color gamut is still fairly limited. With single layer embodiments, pigments are added to colorants in order to expand the range of colors that can be achieved. However, when color results from absorption of electromagnetic radiation, creating colors with combinations of thermochromic materials of limited color range, and pigment colorants, is still quite restrictive in the range of bright colors that can be achieved.
- Additionally, for outdoor usage, thermochromic materials are not only subject to photolytic degradation but by virtue of their sensitivity, need to be protected from mechanical forces such as abrasion, etc.
- Specific thermochromic materials and mixtures of thermochromic materials will need to be adapted to provide the requisite color change. Thermochromic materials usually exist as solids or liquid crystals in a carrier medium.
- When thermochromic materials are used as an optical switch, the theromochromic material needs to be formulated with clear resins and solvents that do not interact. Vibrant color layer formulations using solvents that do not leach are also desired.
- Outdoor usage of thermochromic objects also necessitates good adhesion to substrates and mechanical toughness such as scratch resistance, etc., specific requirements being dictated by the application.
- Accordingly, in view of the above, there is a need for thermochromic materials and thermochromic objects created from such materials that can serve as safety indicators, fashion indicators, or create fun and entertainment by triggering temperature-activated color changes that are vibrant and impactful. The thermochromic objects can also be created in a variety of standard and emissive colors, also with high intensity. The thermochromic objects are also created to minimize photolytic degradation, do not degrade with moisture, and are mechanically robust, particularly in outdoor applications.
- It has now been found that formulations comprising an effective amount of thermochromic materials, at least one liquid carrier medium, at least one polymeric resin, and at least one formulation stabilizing additive, wherein said thermochromic materials are uniformly distributed within said formulation provide thermochromic formulations with high intensity and persistence.
- Accordingly, in one of its formulation aspects, the present invention is directed to a thermochromic formulation comprising an effective amount of thermochromic materials, at least one liquid carrier medium, at least one polymeric resin, and at least one formulation stabilizing additive, wherein said thermochromic materials are uniformly distributed within said formulation.
- In another formulation embodiment, the present invention is directed to a thermochromic formulation comprising an effective amount of thermochromic materials, at least one liquid carrier medium, at least one polymeric resin, and at least one formulation stabilizing additive, wherein said thermochromic materials are uniformly distributed within said formulation and wherein the theromchromic formulation has an FT of at least 90%.
- In one of its object embodiments, the present invention is directed to a thermochromic object comprising a performed article and at least one thermochromic layer, wherein the thermochromic layer results from the foregoing thermochromic formulations.
- In yet another object embodiment, the present invention is directed to a thermochromic object comprising a preformed article, at least one thermochromic layer, and at least one reflective layer, wherein the thermochromic layer results from the foregoing thermochromic formulations, wherein the reflective layer results from a reflective formulation, wherein the reflective layer is proximal to said preformed article, and wherein said thermochromic layer is distal to said preformed article.
- In yet another object embodiment, the present invention is directed to a thermochromic object comprising a preformed article, at least one thermochromic layer, at least one reflective layer, and at least one protective layer, wherein the thermochromic layer results from the foregoing thermochromic formulations, wherein the reflective layer results from a reflective formulation, wherein said protective layer results from a protective formulation, wherein the reflective layer is proximal to said preformed article, and wherein said protective layer is distal to said preformed article, and wherein said thermochromic layer is between said reflective layer and said protective layer.
- In yet another object embodiment, the present invention is directed to a thermochromic object comprising a preformed article, at least one thermochromic layer, and at least one protective layer, wherein the thermochromic layer results from the foregoing thermochromic formulations, wherein the protective layer results from a protective formulation, wherein the thermochromic layer is proximal to said preformed article, and wherein said protective layer is distal to said preformed article.
- In one of its method aspects, the present invention is directed to a method for creating a thermochromic object, said method comprising the steps of obtaining a preformed article and applying to said preformed article at least one thermochromic layer, wherein said thermochromic layer results from the foregoing thermochromic formulations.
- In yet another method embodiment, the present invention is directed to a method for creating a thermochromic object, said method comprising the steps of obtaining a preformed article, applying to said preformed article at least one thermochromic layer, and applying to said preformed article at least one reflective layer, wherein the thermochromic layer results from the foregoing thermochromic formulations, wherein the reflective layer results from a reflective formulation, wherein the reflective layer is proximal to said preformed article, and wherein said thermochromic layer is distal to said preformed article.
- In yet another method embodiment, the present invention is directed to a method for creating a thermochromic object, said method comprising the steps of obtaining a preformed article, applying to said preformed article at least one thermochromic layer, applying to said preformed article at least one reflective layer, and applying to said preformed article at least one protective layer, wherein the thermochromic layer results from the foregoing thermochromic formulations, wherein the reflective layer results from a reflective formulation, wherein said protective layer results from a protective formulation, wherein the reflective layer is proximal to said preformed article, and wherein said protective layer is distal to said preformed article, and wherein said thermochromic layer is between said reflective layer and said protective layer.
- In yet another object embodiment, the present invention is directed to a method for creating a thermochromic object, said method comprising the steps of obtaining a preformed article, applying to said preformed article at least one thermochromic layer, and applying to said preformed article at least one protective layer, wherein the thermochromic layer results from the foregoing thermochromic formulations, wherein the protective layer results from a protective formulation, wherein the thermochromic layer is proximal to said preformed article, and wherein said protective layer is distal to said preformed article.
-
FIG. 1 illustrates how absorption of ultraviolet radiation by a molecule excites it from a vibrational level in the electronic ground state to one of the many vibrational levels in the electronic excited state, such as singlet states and triplet states. -
FIG. 2 is a Jablonski Diagram illustrating processes that occur between the absorption and emission of electromagnetic radiation. -
FIG. 3 is a stylized depiction of an embodiment of the invention whereby a thermochromic object is created using a preformedarticle 1 with athermochromic layer 2 applied thereto. -
FIG. 4 is a stylized depiction of an embodiment of the invention whereby a thermochromic object is created using a preformedarticle 1 with areflective layer 3 and athermochromic layer 2 applied thereto. -
FIG. 5 is a stylized depiction of an embodiment of the invention whereby a thermochromic object is created using a preformedarticle 1 with atheromchromic layer 2 and aprotective layer 4 applied thereto. -
FIG. 6 is a stylized depiction of an embodiment of the invention whereby a thermochromic object is created using a preformedarticle 1 with areflective layer 3, athermochromic layer 2, and aprotective layer 4 applied thereto. -
FIG. 7 is a stylized depiction of an embodiment of the invention whereby transfer technology is used to obtain the thermochromic object. -
FIG. 8 is a stylized depiction of an embodiment of the invention whereby a thermochromic object is created using a preformedarticle 1 with areflective layer 3, a firstthermochromic layer 2, a secondthermochromic layer 5, a firstprotective layer 4, and second protective layer 6 applied thereto. -
FIG. 9 is a stylized depiction of an embodiment of the invention whereby a thermochromic object is created using a preformedarticle 1 with areflective layer 3, a firstthermochromic layer 2, a secondthermochromic layer 5, and a firstprotective layer 4 applied thereto. -
FIG. 10 is a stylized depiction of an embodiment of the invention whereby a thermochromic object is created using a preformedarticle 1 with areflective layer 3, a firstthermochromic layer 2, a firstprotective layer 4, and second protective layer 6 applied thereto. - As noted above, the present invention generally relates to thermochromic formulations, thermochromic objects comprising preformed articles onto which said thermochromic formulations have been applied, and to methods for creating said thermochromic objects.
- Prior to discussing the invention in detail, the following terms will first be defined.
- The term “luminescence” is defined as the emission of electromagnetic radiation from any substance. Luminescence occurs from electronically excited states. As seen in
FIG. 1 , absorption of ultraviolet radiation by a molecule excites it from a vibrational level in the electronic ground state to one of the many vibrational levels in the electronic excited states. The electronic states of most organic molecules can be divided into singlet states and triplet states. - As used herein, “singlet state” refers to when all electrons in the molecule are spin-paired. As used herein, “triplet state” refers to when one set of electron spins is unpaired. The excited state is usually the first excited state. A molecule in a high vibrational level of the excited state will quickly fall to the lowest vibrational level of this state by losing energy to other molecules through collision. The molecule will also partition the excess energy to other possible modes of vibration and rotation.
- “Luminescent materials” are those which emit electromagnetic radiation. Characterizing luminescent materials requires consideration of: (1) the excitation source, (2) the nature of the emission, and (3) whether or not additional stimulation is required to cause emission.
- With regard to the excitation source, luminescent materials excited by electromagnetic radiation are referred to herein as “photoluminescent.” Luminescent materials excited by electrical energy are referred to herein as “electroluminescent.” Luminescent materials excited by a chemical reaction are referred to herein as “chemiluminescent.”
- With regard to the nature of the emission, this may be either fluorescence or phosphorescence. A “fluorescent” material stores electromagnetic radiation and releases it rapidly, in about 10−12 seconds or less. Contrarily, a “phosphorescent” material stores electromagnetic radiation and releases it gradually, in about 10−8 seconds or more.
- Processes that occur between the absorption and emission of electromagnetic radiation are usually illustrated using a Jablonski Diagram, such as the one found in
FIG. 2 . Ground, first, and second electronic states are depicted inFIG. 2 by S0, S1, and S2, respectively. At each electronic energy level, the fluorophores can exist in a number of vibrational energy levels, denoted by 0, 1, 2, etc. Transitions between states are depicted inFIG. 2 as vertical lines to illustrate the instantaneous nature of electromagnetic radiation absorption. - “Fluorescence” occurs when a molecule returns, by emission of a photon, from the excited singlet state to the electronic ground state. If the photon emission occurs between states of the same spin state, that is, from S1 to S0, it is characterized as fluorescence.
- “Phosphorescence” occurs when a molecule goes from the ground state to a metastable state such as T1, a triplet state, and then the metastable state slowly decays back to the ground state S0, via photon emission. Hence, if the spin states between initial and final energy levels are different, that is emission occurs between T1 to S0, it is characterized as phosphorescence.
- The term “thermochromic” is defined as characterizing a change in absorption, reflection, and/or scattering of electromagnetic radiation with a change in temperature to cause a change in the perceived color, wherein the color change may be from colorless to colored, or colored to colorless, or from one color to another.
- “Thermochromic materials” are defined to mean materials that undergo a change in absorption, reflection, and/or scattering of electromagnetic radiation with a change in temperature to cause a change in the perceived color of the materials wherein the color change may be from colorless to colored, or colored to colorless, or from one color to another.
- “Thermochromic objects” are defined to mean objects that undergo a change in absorption, reflection, and/or scattering with a change in temperature to cause a change in the perceived color of the object wherein the color change may be from colorless to colored, or colored to colorless or from one color to another.
- “Negative thermochromism” is defined to describe a temperature-triggered color change resulting from a change in absorption, reflection, and/or scattering wherein the color change is from a colored state to a colorless state.
- “Positive thermochromism” is defined to describe a temperature-triggered color change resulting from a change in absorption, reflection, and/or scattering of electromagnetic radiation wherein the color change is from a colorless to colored state.
- “Neutral thermochromism” is defined to describe a temperature-triggered color change resulting from a change in absorption, reflection, and/or scattering of electromagnetic radiation wherein the color change is from one color to another color.
- “Liquid carrier medium” is a liquid that acts as a carrier for materials distributed in a solid state and/or dissolved therein.
- As used herein, a “formulation” is a liquid carrier medium, as defined above, comprising at least one material either dissolved and/or distributed in a solid state within said liquid carrier medium.
- A “dispersion” is a formulation, as defined above, wherein said material is a solid distributed in the liquid carrier medium.
- A “thermochromic formulation” is a formulation, as defined above, which additionally comprises thermochromic materials as defined above.
- As used herein, a “thermochromic layer” is a film resulting from at least one thermochromic formulation that is substantially dry, as characterized by the residual liquid carrier medium being in the range of 1-5 weight % of the total weight of the film.
- A “reflective formulation” is a formulation, as defined above, which comprises at least a polymeric resin in a liquid carrier medium, as defined above, and further comprises at least one colorant (white or non-white).
- As used herein, a “reflective layer” is a film resulting from at least one reflective formulation that is substantially dry, as characterized by the residual liquid carrier medium being in the range of 1-5 weight % of the total weight of the film.
- A “white reflectance layer” is one that reflects 95% of visible electromagnetic radiation incident upon it.
- A “protective formulation” is a formulation, as defined above, which comprises at least a polymeric resin selected for environmental or mechanical protection of the underlying article, upon application onto said article.
- A “protective layer” is a film resulting from at least one protective formulation that is substantially dry, as characterized by the residual liquid carrier medium being in the range of 1-5 weight % of the total weight of the film.
- A “stabilizing additive” is a material added to a formulation comprising solid particles or a dispersion to uniformly distribute, prevent agglomeration, and/or prevent settling of solid materials in said dispersion in said liquid carrier medium to result in an enhancement of luminous intensity. Such stabilizing additives generally comprise dispersants and/or rheology modifiers.
- A “preformed article” is any article onto which at least one layer may be applied. A preformed article may be rigid or flexible.
- As used herein, “visible electromagnetic radiation” is characterized by electromagnetic radiation with wavelengths in the region of 400 nanometers (“nm”) to 700 nm.
- As used herein, “Film Transmissivity” (“FT”) is the fraction of incident visible electromagnetic radiation transmitted through a layer.
- “Photolytic degradation” is deterioration, degradation, or change in properties, such as observed color, that is initiated by electromagnetic radiation.
- The present invention relates to thermochromic materials that are formulated to be liquid mixtures with stabilizing additives comprising polymeric resin binders, dispersants, rheology modifiers, and wetting agents so as to be highly transmissive of visible electromagnetic radiation in the colorless thermochromic state upon application to any article.
- The present invention also relates to the use of photoluminescent fluorescent pigment materials to render color or aid in rendering color upon triggering of thermochromic materials into a colorless state.
- The present invention also relates to the use of the luminescent fluorescent dye materials such that they exist as solid solutions in the polymeric resin matrix, thereby creating high ratio of emission per unit weight of material deployed for rendering color or aid in rendering color upon triggering of the thermochromic materials into a colorless state
- The present invention also relates to the use of photostabilizers either singly or in combination or with a combined functionality in a single molecule to retard the photolytic degradation of said thermochromic and photoluminescent fluorescent materials cited above.
- The present invention also relates to a thermochromic objects comprising at least one thermochromic layer, wherein said thermochromic layer is highly transmissive of visible electromagnetic radiation and wherein the thermochromic materials have been triggered into a colorless state.
- The present invention also relates to a thermochromic objects comprising at least one thermochromic layer, as defined above, but which may additionally comprise photoluminescent fluorescent pigments, dyes, or both, as described above.
- The present invention also relates to the construction of a thermochromic objects comprising at least one thermochromic layer, as defined abov,e but which may additionally comprises photostabilizers, as described above
- The present invention also relates to the construction of a thermochromic object which comprises a thermochromic layer, as defined above, wherein the thermochromic layer functions as an optical switch to transition from either a colored state to a colorless state or from a colorless state to a colored state.
- The present invention also relates to a thermochromic object which comprises a reflective layer comprising polymeric resin and white or colored pigments, wherein the reflective layer is highly reflective of unabsorbed visible electromagnetic radiation and wherein such reflective layer is deployed in conjunction with a thermochromic layer.
- The present invention also relates to a thermochromic objects comprising a reflective layer, as defined above, and additionally comprising photoluminescent fluorescent pigments wherein the reflective layer is highly reflective of unabsorbed visible electromagnetic radiation.
- The present invention also relates to a thermochromic object comprising a reflective layer, as defined abov,e and additionally comprising photoluminescent fluorescent dyes wherein the photoluminescent fluorescent dye exists as a solid solution in polymeric matrix wherein the reflective layer is highly reflective of unabsorbed visible electromagnetic radiation.
- The present invention also relates to a thermochromicobject comprising a reflective layer as defined above which additionally comprises photostabilizers as defined above to retard the photolytic degradation of said thermochromic and photoluminescent fluorescent materials cited above
- When the thermochromic object according to the invention comprises at least one reflective layer and at least one thermochromic layer, the reflective layer is applied first, so that it is proximal to the preformed article. The thermochromic layer is applied after the reflective layer, so that said thermochromic layer is distal to said preformed article. If transfer technology is used, it must be used such that the result is the same.
- The present invention also relates to a thermochromic object comprising at least one protective layer which comprises a polymeric resin and wherein the protective layer is deployed in conjunction with an thermochromic layer and wherein the protective layer provides physical protection to the thermochromic layer.
- The present invention also relates to a thermochromic object comprising at least one protective layer as defined above but which additionally comprises photostabilizers as described above.
- When the thermochromic object according to the invention comprises at least one thermochromic layer and at least one protective layer, the thermochromic layer is applied first, so that it is proximal to said preformed article. The protective layer is applied after the thermochromic layer, so that said protective layer is distal to said preformed article. If transfer technology is used, it must be used such that the result is the same.
- When the thermochromic object according to the invention comprises at least one reflective layer, at least one thermochromic layer, and at least one protective layer, the reflective layer is applied first, so that it is proximal to said preformed article. The thermochromic layer is applied next and then the protective layer is applied. Therefore, the reflective layer is proximal to the preformed article, the protective layer is distal to said preformed article, and said thermochromic layer is between said reflective layer and said protective layer. If transfer technology is used, it must be used such that the result is the same.
- The present invention also relates to the method of making a thermochromic object with at least one thermochromic layer.
- The present invention also relates to the method of making a thermochromic object with at least one reflective layer and one thermochromic layer.
- The present invention also relates to the method of making a thermochromic object with at least one thermochromic layer and at least one protective layer.
- The present invention also relates to the method of making a thermochromic object with at least one colorant layer and at least one thermochromic layer.
- The present invention relates to the method of making a thermochromic object with at least one reflective layer, at least one thermochromic layer and at least one protective layer.
- The present invention relates to the method of making a thermochromic object with at least one colorant layer, at least one thermochromic layer and at least one protective layer.
- The present invention relates to the method of making a thermochromic object with at least one reflective layer, at least one colorant layer, at least one thermochromic layer and at least one protective layer.
- In order to produce these products, one needs to develop a system construction such that the visual impact of the color change is striking, the indicator indicia are robust, the applied images patterns or indicia are photolytically stable and the product is not only durable for the user but also environmentally stable. One also needs to develop products that are desirable and useful to consumers in many fields of use, including food products, entertainment, sports, transportation, weather protection, decorating, indoor and outdoor sanitation or a combination thereof.
- New products have surprisingly been developed that address the problems associated with various compounds and their uses, including developing products which have color changing indicators based on thermochromic, fluroscent photochromic, phosphorescent photochromic compounds or a combination thereof and that are constructed from a variey of substrate materials such as PVC acrylics, urethanes polyester, nylon, etc. The substrate or surface construction materials or components may be rigid or flexible.
- A system construction has also been developed such that the visual impact of the color change is striking, the indicator indicia are robust, the applied images patterns or indicia are photolytically stable and the product is not only durable for the user but also environmentally stable. These new products are also desirable and useful to consumers in many fields of use, including food products, entertainment, sports, transportation, weather protection, decorating, indoor and outdoor sanitation or a combination thereof. Specifically, in contemplated embodiments, a multilayer system construction or layered material has been designed so as to create a visually striking impact of the color change, to be durable with respect to scratches and abrasions and to have weatherometric robustness, such as photolytic stability and atmospheric stability for the indicator indicia or images.
- One contemplated embodiment comprises a three functional layer structure with a base layer providing functionality for maximizing or that maximizes the desired visual impact of the color change of at least part of the layered material, a thermochromic layer providing functionality for triggering a state change that is, either colorless to colored, or colored to colorless, or from one color to another, and a protective layer that provides functionality for handling and weatherometric robustness for the underlying image. The protective layer can also provide a visual enhancement function when desired by providing a reflective component or gloss for image viewing.
- The base functional layer may produce the desired functionality either by a single or multiple layers of material, depending on the application and product. The base layer may be constructed either by blending the final color dye or pigment before fabrication of the layered material, such as by extrusion or molding or formation of plastic, glass, paper etc., or by a coating application process such as gravure, flexo, roll or blade coating etc., or by printing application processes such as screen printing or pad printing etc. One example of a base layer construction would to apply a layer containing a dye or pigment material including one or more fluorescent dyes or pigments that will render a brilliant final color. In this type of application, the thermochromic layer (applied on top of this layer) would generally function as an optical switch going from colored to colorless. This indicator could be accomplished by the base layer having the appropriate dye or pigment for the desired visual impact. Another application of a base layer construction is where the base layer contains just a white reflective pigment coating to maximze the color rendering of the thermochromic materials. In this type of application the thermochromic layer would go from either colorless to colored or from one color to another.
- As stated earlier, the base layer may also comprise multiple layers. An example of a two layer base functional layer is contemplated when the base layer is being applied to a rigid or flexible plastic or other material containing a strong absorbing color different from the final color. In such a case so as not to reduce final color impact or vibrancy, the base layer will consist of a hidden layer consisting of a white reflective pigment application followed by the layer rendering the final color such as that described above.
- There will be occasions where in order to create a visually-striking final color, it may be necessary to first apply a specific contrasting color layer to enable striking visual impact of the final color. In such an embodiment, the base functional layer could become a three layer construction. In this case, one has to first create a desired oversized (oversized relative to final color image) contrast color image followed by a white reflective image followed by final color image application.
- The base layer, in addition to serving the function of enhancing the visual impact of the final color, will also serve as a platform for the additional layers and/or functional layers. Therefore, it is important to consider the components of the base layer with respect to the components of additional layers in order to avoid color bleeding, undesirable chemical reactions between layers of materials and deterioration of the desired visual impact of the layered materials.
- The second layer applied on top of the base layer will contain the thermochromic layer. The thermochromic layer may have one or more layers, where the layer or layers comprise at least one layer of envirochromic dyes, pigments or inks. Envirochromic dyes, pigments or inks are those that change color or texture with a changing environment, such as heat, cold, rain, sunshine, UV rays, snow, dark, light or a combination thereof. Envirochromic dyes, pigments or inks are contemplated to be those described herein earlier, conventional envirochromic dyes, pigments or inks, envirochromic dyes, pigments or inks that are yet to be developed or a combination thereof. Envirochromic inks, dyes and pigments may be clear until triggered, opaque until triggered or a particular color until triggered. Once triggered by an environmental condition, the ink, dye or pigment will change—whether it changes from clear to opaque or colored, from opaque to clear or colored or from colored to opaque or clear.
- Dispersing Agents
- High mechanical forces are necessary to incorporate solids in liquid media. It is customary to employ “dispersing agents” in order to reduce these dispersion forces and in order to keep the total energy input into the system, which is necessary for deflocculating the solid particles and thus the time of dispersion, as low as possible. These dispersing agents are surface-active substances of anionic, cationic, or neutral structure. These substances are added in a small amount, either directly to the solid or to the dispersion medium. Furthermore, it is known that even after complete deflocculation of the solid agglomerates into primary particles, re-agglomeration occurs after the dispersion process. In such a case, the effort expended to produce a dispersion is partially or completely negated.
- There is a multiplicity of different substances which are used nowadays as dispersing agents for pigments and extenders. A review of the existing patent literature is given in European Patent No. 0 318 999. See, e.g.,
Page 2, Lines 24-26. Apart from very simple, low molecular weight compounds such as lecithin, fatty acids, and salts thereof, and alkylphenol ethoxylates, for example, complex structures are also used as dispersing agents. In particular, these comprise amino- and amide-functional systems, which are widely used amongst dispersing agents. In British Patent No. 2 153 804, for example, amino- and amide-functional poly- and oligocopolymers based on polyamines and polycaprolactones are used for the dispersion of magnetic pigments. European Patent No. 0 713 894 describes the use of amino-functional polylactones for coatings and printing inks. Moreover, amine-functional polyacrylates, such as those disclosed in European Patent No. 0 311 157 and in U.S. Pat. No. 3,980,602 are used for the stabilisation of organic and inorganic pigments. Amine-functional polymers based on polyisocyanates constitute a further group. See, e.g., European Patent Nos. 0 159 678 and 0 438 836. - Derivatives of phosphoric acid esters are also frequently used as dispersing agents. European Patent No. 0 417 490, see
Page 2, Lines 23-43, gives a summary of the use of these substances, preferably as dispersing agents or for the pretreatment of pigments. The salts of acidic phosphoric acid esters are also described in this patent. Inorganic bases as well as mono- and diamines are listed as the basic salt formation components. - While satisfactory stabilization of pigments or solids can be achieved with one or more of the dispersing aids cited above, many of these dispersing agents have an insufficient capacity for reducing the viscosity on the incorporation of pigments or of solid particles in binder vehicles. Additionally, for manufacturing efficiency, there is a need to minimize the thickness of the thermochromic layer together with the necessity of reducing the amount of solvent as far as possible (e.g., high-solids formulations). All of this can lead to high viscosity with the resulting need for the application of excessive energy to disperse the pigment. This can result in significant degradation.
- Examples of suitable dispersing aids that minimize agglomeration require very low levels of energy are cited for example an acrylic acid-acrylamide polymer such as those cited in U.S. Pat. No. 6,596,816, incorporated herein by reference for all purposes, or salts of an amine functional compound and an acid such as those cited in U.S. Pat. No. 6,111,054, also incorporated herein by reference for all purposes.
- Rheology Modifiers
- “Rheology Modifiers” are those substances which generally can build viscosity in liquid dispersion formulations, thereby retarding settling of pigment materials while at the same time significantly lowering viscosity upon application of shear, to enhance smooth applicability of such formulations onto articles. There is a widespread practice of using materials such as colloidal silica or fumed silica and magnesium aluminum silicate clays, such as bentonite, not only as thixotropic modifiers to prevent sagging and running of luminescent formulation as it is applied to the object but also as suspending fillers, that is, for minimizing settling of dense pigment particles such as phosphor particles. See, e.g., U.S. Pat. No. 6,207,077.
- The common practice is to use organically-modified bentonites, silicas, hydrogenated castor oil, and polyamide waxes. A disadvantage of these substances is that they are mostly dry solids which have to be brought into the form of a semi-finished product using solvents and shear forces, and incorporated into the liquid coating system under careful temperature control. Failure to observe such temperatures results in crystallites in the finished coating system, which may lead to defects in the coating.
- The bigger disadvantage of their use in luminescent coatings such as the thermochromic layer is that they lead to turbidities and haze rather than transparent coatings. Moreover, handling dry pulverulent products which give rise to dusts in the course of processing is undesirable.
- The present invention employs polymeric urea-urethanes in aprotic polar solvents as rheology modifiers. This class of rheology modifiers can be used satisfactorily, that is without any scattering of electromagnetic radiation, and without any significant build up of viscosity. Thus, this class serves not only as rheology modifiers but also to minimize settling of the dense pigment particles. Examples of such urea-urethanes can be found, for example, in U.S. Pat. No. 6,617,468 and U.S. Pat. No. 6,870,024, incorporated herein by reference for all purposes.
- Wetting Agents
- If the applied luminescent envirochromic layer does not contain “wetting agents,” also known as leveling agents, the resulting surface may not be smooth. Instead, the surface may be less structured, referred to as having a wavy surface or as having an orange peel-like surface. These surfaces may be finely structured, with a short wave, or coarsely structured, with a long wave.
- This waviness is unwanted not only because the surface is not visually appealing with a lowered market appeal, but more importantly, any surface structure is likely to cause light scattering and loss of perceived luminous intensity.
- The structure depends on the nature and composition of the coating compositions; for example, on whether these coating compositions comprise solvents or else are solvent-free, as in the case of powder coating materials. In the case of powder coating materials it is absolutely necessary to add leveling agents, since without these leveling agents it is impossible to achieve a surface which is in any way smooth.
- Known examples of such agents are poly(meth)acrylates and polysiloxanes, which may be used as leveling promoters for coatings. In the case of the polysiloxanes, the compounds generally comprise polydimethylsiloxanes, polymethylalkylsiloxanes, or else polyether- or polyester-modified polydimethyl- or polymethylalkylsiloxanes. In the case of the poly(meth)acrylates, preference is given to the use of polymers or copolymers of alkyl acrylates having an alkyl radical chain length of C2-C8, such as ethyl acrylate, 2-ethylhexyl acrylate, or n-butyl acrylate, for example. The products used possess in some cases molecular weights of up to 100,000.
- The action of all these products is based on surface activity at the liquid/gas interface: owing to a certain incompatibility with the actual binder of the coating system, these products adopt an orientation to the interface. This incompatibility may be increased by raising the molecular weight of these polymers. A disadvantage then, however, is that owing to this incompatibility there can be cases wherein the scattering of electromagnetic radiation or haze of the layer becomes high, thereby resulting in significant reduction in luminous intensity. The present invention employs branched polymers comprising a free-radically or ionically polymerized base molecule into which monoethylenically unsaturated macromonomeric units have been incorporated by copolymerization. Examples of such polymers may be found in U.S. Pat. No. 6,710,127, incorporated herein by reference for all purposes.
- Other Additives
- Other additives, such as “deaerators” and “defoamers” may be employed. Deaerators are those substances which minimize entrained air. Defoamers are those substances that allow easier dissipation of entrained air. Depending upon the materials comprising the formulation and the resultant viscosity, a significant amount of air entrainment can cause scattering of electromagnetic radiation and, hence, a reduction in luminous intensity.
- The following examples are offered to illustrate the present invention and should not be construed in any way as limiting the scope of the invention.
- A formulation was made consisting of a thermochromic pigment in a binder of nitrocellulose and diluted with 2-methoxyethanol. This gave pigment solids of 40% of dry pigment to 60% Nitrocellulose binder. The solids were contained in a solvent mixture at 45% solids. This material was coated over plasticized PVC to give a dry coating thickness of 18 microns using a screen printing process. The color of the thermochromic pigment at room temperature was selected so that it contrasted the color of the PVC. The opacity of the thermochromic layer at room temperature masked the PVC layer below it. The thermochromic layer became translucent when heated through normal body contact to reveal the contrasting base color of the PVC underneath.
- A formulation was made consisting of 52% thermochromic pigment in 48% Nitrocellulose binder. The pigment and binder were prepared in a solvent mixture containing Toluene, Hexane, and Hydrocarbon solvents at a solids content of 40%. This material was applied using a silk screen to give a dry coating thickness of 22 microns.
- A yellow fluorescent pigment was dispersed in 2-methoxyethanol at 50% solids using the wetting agent TegoWet 550. This dispersion was added to an acrylic binder (NeoCryl B-735) to give solids of 20% in 2-methoxyethanol. The ink was then applied to plasticized PVC using a screen print to achieve a 15 micron thickness, which provided for an image that contrasted the base color of the PVC. Over this was applied the formulation from Example 2 at a thickness of 26 microns. The color of the thermochromic layer at room temperature contrasted with the fluorescent layer below it. In this case, the thermochromic layer was dark blue compared to the fluorescent yellow of the layer below the thermochromic layer. The opacity of the thermochromic layer masked the fluorescent layer below it. The image was such that when warmed, the thermochromic pigment became translucent and revealed the fluorescent layer printed underneath.
- Thermochromic pigment was mixed with 10% 2-methoxyethanol and then dispersed in plastisol to give a pigment concentration of 40% in the final mixture. This was then applied over a polyester fabric using a applicator to give a 1 mil ending thickness.
Claims (20)
1. A thermochromic formulation comprising:
(a) an effective amount of thermochromic materials;
(b) at least one liquid carrier medium;
(c) at least one polymeric resin; and
(d) at least one stabilizing additive,
wherein said thermochromic materials are uniformly distributed within said formulation.
2. A thermochromic formulation comprising:
(a) an effective amount of thermochromic materials;
(b) at least one liquid carrier medium;
(c) at least one polymeric resin; and
(d) at least one stabilizing additive,
wherein said thermochromic materials are uniformly distributed within said formulation and further wherein said formulation has an FT of at least 90%.
3. The thermochromic formulation of claim 1 or claim 2 , which further comprises electromagnetic radiation absorptive pigment materials.
4. The thermochromic formulation of claim 1 or claim 2 , which further comprises photoluminescent fluorescent pigment materials.
5. The thermochromic formulation of claim 4 , wherein said photoluminescent fluorescent pigment materials are selected such that they are in solution in said liquid carrier medium and wherein upon application of said theromochromic layer said photoluminescent fluorescent pigment materials exist as a solid state solution said polymeric resin matrix.
6. The photoluminescent formulation of any one of claims 3-5, which further comprises photostabilizers.
7. The photoluminescent formulation of any one of claims 1-6, wherein said thermochromic materials are negative thermochromic materials.
8. The photoluminescent formulation of any one of claims 1-6, wherein said thermochromic materials are positive thermochromic materials.
9. The photoluminescent formulation of any one of claims 1-6, wherein said thermochromic materials are neutral thermochromic materials.
10. The photoluminescent formulation of claim 7 or claim 8 , wherein said thermochromic materials comprise an electron donating compound and an electron accepting compound.
11. The photoluminescent formulation of claim 7 or claim 8 , wherein said thermochromic materials comprise liquid crystal materials.
12. The thermochromic formulation of any one of claims 1-6, wherein said polymeric resin is selected from the group consisting of acrylates, poly vinyl chloride, polyurethanes, polycarbonates, polyesters, and combinations thereof.
13. A thermochromic object comprising:
(a) a preformed article; and
(b) at least one thermochromic layer
wherein said thermochromic layer results from a formluation of any of claims 1-12.
14. A thermochromic object comprising:
(a) a preformed article;
(b) at least one thermochromic layer; and
(c) at least one reflective layer;
wherein said thermochromic layer results from a formluation of any of claims 1-12, wherein said reflective layer results from a reflective formulation, wherein said reflective layer is proximal to said preformed article, and wherein said thermochromic layer is distal to said preformed article.
15. A thermochromic object comprising:
(a) a preformed article;
(b) at least one thermochromic layer;
(c) at least one reflective layer; and
(d) at least one protective layer;
wherein said thermochromic layer results from a formulation of any of claims 1-12, wherein said reflective layer results from a reflective formulation, wherein said protective layer results from a protective formulation, wherein said reflective layer is proximal to said preformed article, wherein said protective layer is distal to said preformed article, and wherein said thermochromic layer is between said reflective layer and said protective layer.
16. A thermochromic object comprising:
(a) a preformed article;
(b) at least one thermochromic layer; and
(c) at least one protective layer;
wherein said thermochromic layer results from a formluation of any of claims 1-12, wherein said protective layer results from a protective formulation, wherein said thermochromic layer is proximal to said preformed article, and wherein said protective layer is distal to said preformed article.
17. A method for creating a thermochromic object, said method comprising the steps of:
(a) obtaining a preformed article; and
(b) applying to said preformed article at least one thermochromic layer
wherein said thermochromic layer results from a formulation of any one of claims 1-12.
18. A method for creating a thermochromic object, said method comprising the steps of:
(a) obtaining a preformed article;
(b) applying to said preformed article at least one thermochromic layer; and
(c) applying to said preformed article at least one reflective layer;
wherein said thermochromic layer results from a formluation of any of claims 1-12, wherein said reflective layer results from a reflective formulation, wherein said reflective layer is proximal to said preformed article, and wherein said thermochromic layer is distal to said preformed article.
19. A method for creating a thermochromic object, said method comprising the steps of:
(a) obtaining a preformed article;
(b) applying to said preformed article at least one thermochromic layer;
(c) applying to said preformed article at least one reflective layer; and
(d) applying to said preformed article at least one protective layer;
wherein said thermochromic layer results from a formulation of any of claims 1-12, wherein said reflective layer results from a reflective formulation, wherein said protective layer results from a protective formulation, wherein said reflective layer is proximal to said preformed article, wherein said protective layer is distal to said preformed article, and wherein said thermochromic layer is between said reflective layer and said protective layer.
20. A method for creating a thermochromic object, said method comprising the steps of:
(a) obtaining a preformed article;
(b) applying to said preformed article at least one thermochromic layer; and
(c) applying to said preformed article at least one protective layer;
wherein said thermochromic layer results from a formulation of any of claims 1-12, wherein said protective layer results from a protective formulation, wherein said thermochromic layer is proximal to said preformed article, and wherein said protective layer is distal to said preformed article.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11/311,289 US20060159925A1 (en) | 2004-12-20 | 2005-12-20 | High-intensity, persistent thermochromic compositions and objects, and methods for creating the same |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US63753504P | 2004-12-20 | 2004-12-20 | |
| US11/311,289 US20060159925A1 (en) | 2004-12-20 | 2005-12-20 | High-intensity, persistent thermochromic compositions and objects, and methods for creating the same |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20060159925A1 true US20060159925A1 (en) | 2006-07-20 |
Family
ID=36602251
Family Applications (8)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/311,289 Abandoned US20060159925A1 (en) | 2004-12-20 | 2005-12-20 | High-intensity, persistent thermochromic compositions and objects, and methods for creating the same |
| US11/311,290 Abandoned US20060172135A1 (en) | 2004-12-20 | 2005-12-20 | Layered envirochromic materials, applications and methods of preparation thereof |
| US11/793,376 Active 2028-12-15 US8163201B2 (en) | 2004-12-20 | 2005-12-20 | High-intensity, persistent photoluminescent formulations and objects, and methods for creating the same |
| US12/874,441 Abandoned US20110012062A1 (en) | 2004-12-20 | 2010-09-02 | High-intensity, persistent photoluminescent formulations and objects, and methods for creating the same |
| US13/115,843 Active US8287757B2 (en) | 2004-12-20 | 2011-08-03 | High-intensity, persistent photoluminescent formulations and objects, and methods for creating the same |
| US13/115,850 Active US8282858B2 (en) | 2004-12-20 | 2011-08-05 | High-intensity, persistent photoluminescent formulations and objects, and methods for creating the same |
| US13/115,854 Active US8293136B2 (en) | 2004-12-20 | 2011-08-05 | High-intensity, persistent photoluminescent formulations and objects, and methods for creating the same |
| US13/523,923 Active - Reinstated US8409662B2 (en) | 2004-12-20 | 2012-06-15 | High-intensity, persistent photoluminescent formulations and objects, and methods for creating the same |
Family Applications After (7)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/311,290 Abandoned US20060172135A1 (en) | 2004-12-20 | 2005-12-20 | Layered envirochromic materials, applications and methods of preparation thereof |
| US11/793,376 Active 2028-12-15 US8163201B2 (en) | 2004-12-20 | 2005-12-20 | High-intensity, persistent photoluminescent formulations and objects, and methods for creating the same |
| US12/874,441 Abandoned US20110012062A1 (en) | 2004-12-20 | 2010-09-02 | High-intensity, persistent photoluminescent formulations and objects, and methods for creating the same |
| US13/115,843 Active US8287757B2 (en) | 2004-12-20 | 2011-08-03 | High-intensity, persistent photoluminescent formulations and objects, and methods for creating the same |
| US13/115,850 Active US8282858B2 (en) | 2004-12-20 | 2011-08-05 | High-intensity, persistent photoluminescent formulations and objects, and methods for creating the same |
| US13/115,854 Active US8293136B2 (en) | 2004-12-20 | 2011-08-05 | High-intensity, persistent photoluminescent formulations and objects, and methods for creating the same |
| US13/523,923 Active - Reinstated US8409662B2 (en) | 2004-12-20 | 2012-06-15 | High-intensity, persistent photoluminescent formulations and objects, and methods for creating the same |
Country Status (8)
| Country | Link |
|---|---|
| US (8) | US20060159925A1 (en) |
| EP (1) | EP1833943A4 (en) |
| JP (1) | JP2008524401A (en) |
| AU (1) | AU2005319365B2 (en) |
| CA (1) | CA2591803A1 (en) |
| MX (1) | MX2007007445A (en) |
| SG (1) | SG159492A1 (en) |
| WO (1) | WO2006069028A2 (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20120080615A1 (en) * | 2010-09-30 | 2012-04-05 | Performance Indicator, Llc. | Photolytically and environmentally stable multilayer structure for high efficiency electromagentic energy conversion and sustained secondary emission |
| CN110791190A (en) * | 2019-10-30 | 2020-02-14 | 湖南松井新材料股份有限公司 | Water-based self-luminous elastic coating and preparation method and application thereof |
Families Citing this family (253)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7910022B2 (en) * | 2006-09-15 | 2011-03-22 | Performance Indicator, Llc | Phosphorescent compositions for identification |
| US20060159925A1 (en) | 2004-12-20 | 2006-07-20 | Satish Agrawal | High-intensity, persistent thermochromic compositions and objects, and methods for creating the same |
| US20110140002A1 (en) * | 2004-12-20 | 2011-06-16 | Performance Indicator, Llc | Photoluminescent Compositions, Methods of Manufacture and Novel Uses |
| US8092866B2 (en) * | 2005-03-22 | 2012-01-10 | World Wide Lines, Inc. | Thermochromatic pigment covered article and method of making the same |
| EP1880236B1 (en) * | 2005-05-10 | 2018-10-03 | DataTrace DNA Pty Ltd | High-resolution tracking of industrial process materials using trace incorporation of luminescent markers |
| US7547894B2 (en) * | 2006-09-15 | 2009-06-16 | Performance Indicator, L.L.C. | Phosphorescent compositions and methods for identification using the same |
| JP5428147B2 (en) * | 2006-12-07 | 2014-02-26 | 三菱化学株式会社 | Organic phosphor material |
| US7684997B2 (en) * | 2006-12-27 | 2010-03-23 | Pitney Bowes Inc. | Machine readable colored envelopes |
| US8029190B2 (en) * | 2007-05-10 | 2011-10-04 | Kimberly-Clark Worldwide, Inc. | Method and articles for sensing relative temperature |
| US20090060165A1 (en) * | 2007-08-30 | 2009-03-05 | Pradeep Kumar Dani | Method and System for Customer Transaction Request Routing |
| FR2933100B1 (en) | 2008-06-25 | 2010-08-13 | Commissariat Energie Atomique | RUMINESCENT RARE EARTH OXIDE PARTICLE DISPERSIONS, VARNISH COMPRISING THESE PARTICLES, PROCESSES FOR PREPARING THE SAME, AND METHOD FOR MARKING SUBSTRATES. |
| CA2744538A1 (en) * | 2008-11-25 | 2010-06-17 | David Postma | Luminescent paints and methods of making the same |
| US8523645B2 (en) * | 2009-03-25 | 2013-09-03 | Nike, Inc. | Golf club head and head cover combination providing enhanced functionality |
| WO2010114810A1 (en) * | 2009-04-01 | 2010-10-07 | David Postma | Luminescent paints and methods of making the same |
| US7960688B2 (en) * | 2009-06-18 | 2011-06-14 | Performance Indicator Llc | Photoluminescent markings with functional overlayers |
| WO2011053412A1 (en) * | 2009-10-30 | 2011-05-05 | Defense Holdings, Inc. | Method of illuminating a magnetic compass or other type of indicia in low light situations using photoluminescent materials |
| JP5712550B2 (en) * | 2010-10-12 | 2015-05-07 | 日立化成株式会社 | Spherical phosphor, wavelength conversion type solar cell encapsulant, solar cell module, and method for producing them |
| US8664624B2 (en) | 2010-09-30 | 2014-03-04 | Performance Indicator Llc | Illumination delivery system for generating sustained secondary emission |
| TWI494639B (en) * | 2010-12-08 | 2015-08-01 | Ind Tech Res Inst | Camouflage structure capable of altering its appearance |
| US8404153B2 (en) | 2010-12-17 | 2013-03-26 | General Electric Company | White persistent phosphor blend or layered structure |
| US8506843B2 (en) | 2010-12-17 | 2013-08-13 | General Electric Company | White emitting persistent phosphor |
| WO2012145648A1 (en) * | 2011-04-22 | 2012-10-26 | Once Innovations, Inc. | Extended persistence and reduced flicker light sources |
| US20130286461A1 (en) * | 2012-04-27 | 2013-10-31 | Pleotint, L.L.C. | Synergistic reversible chromism |
| WO2013173451A2 (en) * | 2012-05-16 | 2013-11-21 | Krook Justin | Photoluminescent compositions and uses thereof |
| US8952341B2 (en) | 2012-09-06 | 2015-02-10 | Performance Indictor, LLC | Low rare earth mineral photoluminescent compositions and structures for generating long-persistent luminescence |
| US9057021B2 (en) | 2012-09-06 | 2015-06-16 | Performance Indicator, Llc | Photoluminescent objects |
| US20140072738A1 (en) * | 2012-09-10 | 2014-03-13 | Throw Glow, LLC | Sporting equipment covering |
| US9868863B1 (en) * | 2013-02-08 | 2018-01-16 | Swift IP, LLC | Compositions having slip resistance and luminous properties |
| US9121557B2 (en) | 2013-03-15 | 2015-09-01 | Jianqiao YANG | Lamp having multi-functional support |
| US9206382B2 (en) | 2013-05-28 | 2015-12-08 | The Procter & Gamble Company | Surface treatment compositions comprising photochromic dyes |
| EP2813295A1 (en) * | 2013-06-13 | 2014-12-17 | Stanley Works (Europe) GmbH | Hand tool having a fluorescent PVC coating |
| US9839098B2 (en) | 2013-11-21 | 2017-12-05 | Ford Global Technologies, Llc | Light assembly operable as a dome lamp |
| US9464803B2 (en) | 2013-11-21 | 2016-10-11 | Ford Global Technologies, Llc | Illuminated speaker |
| US9499096B2 (en) | 2013-11-21 | 2016-11-22 | Ford Global Technologies, Llc | Photoluminescent vehicle reading lamp |
| US9464776B2 (en) | 2013-11-21 | 2016-10-11 | Ford Global Technologies, Llc | Vehicle light system with illuminating exhaust |
| US9688186B2 (en) | 2013-11-21 | 2017-06-27 | Ford Global Technologies, Llc | Illuminating decal for a vehicle |
| US9931991B2 (en) | 2013-11-21 | 2018-04-03 | Ford Global Technologies, Llc | Rotating garment hook |
| US9492575B2 (en) | 2013-11-21 | 2016-11-15 | Ford Global Technologies, Llc | Color changing and disinfecting surfaces |
| US9499090B2 (en) | 2013-11-21 | 2016-11-22 | Ford Global Technologies, Llc | Spoiler using photoluminescent illumination |
| US9538874B2 (en) | 2013-11-21 | 2017-01-10 | Ford Global Technologies, Llc | Photoluminescent cupholder illumination |
| US9688192B2 (en) | 2013-11-21 | 2017-06-27 | Ford Global Technologies, Llc | Vehicle having interior and exterior lighting on tailgate |
| US9463738B2 (en) | 2013-11-21 | 2016-10-11 | Ford Global Technologies, Llc | Seatbelt lighting system |
| US10041650B2 (en) | 2013-11-21 | 2018-08-07 | Ford Global Technologies, Llc | Illuminated instrument panel storage compartment |
| US9539939B2 (en) | 2013-11-21 | 2017-01-10 | Ford Global Technologies, Llc | Photoluminescent logo for vehicle trim and fabric |
| US9905743B2 (en) | 2013-11-21 | 2018-02-27 | Ford Global Technologies, Llc | Printed LED heat sink double lock |
| US9810401B2 (en) | 2013-11-21 | 2017-11-07 | Ford Global Technologies, Llc | Luminescent trim light assembly |
| US9586523B2 (en) | 2013-11-21 | 2017-03-07 | Ford Global Technologies, Llc | Vehicle lighting assembly |
| US9573517B2 (en) | 2013-11-21 | 2017-02-21 | Ford Global Technologies, Llc | Door illumination and warning system |
| US9463736B2 (en) | 2013-11-21 | 2016-10-11 | Ford Global Technologies, Llc | Illuminated steering assembly |
| US9969323B2 (en) | 2013-11-21 | 2018-05-15 | Ford Global Technologies, Llc | Vehicle lighting system employing a light strip |
| US9950658B2 (en) | 2013-11-21 | 2018-04-24 | Ford Global Technologies, Llc | Privacy window system |
| US9487127B2 (en) | 2013-11-21 | 2016-11-08 | Ford Global Technologies, Llc | Photoluminescent vehicle step lamp |
| US9539941B2 (en) | 2013-11-21 | 2017-01-10 | Ford Global Technologies, Llc | Photoluminescent cupholder illumination |
| US9487135B2 (en) | 2013-11-21 | 2016-11-08 | Ford Global Technologies, Llc | Dome light assembly |
| US9587800B2 (en) | 2013-11-21 | 2017-03-07 | Ford Global Technologies, Llc | Luminescent vehicle molding |
| US10363867B2 (en) | 2013-11-21 | 2019-07-30 | Ford Global Technologies, Llc | Printed LED trim panel lamp |
| US9463737B2 (en) | 2013-11-21 | 2016-10-11 | Ford Global Technologies, Llc | Illuminated seatbelt assembly |
| US9499092B2 (en) | 2013-11-21 | 2016-11-22 | Ford Global Technologies, Llc | Illuminating molding for a vehicle |
| US9771019B2 (en) | 2013-11-21 | 2017-09-26 | Ford Global Technologies, Inc. | Photoluminescent vehicle illumination |
| US9809160B2 (en) | 2013-11-21 | 2017-11-07 | Ford Global Technologies, Llc | Tailgate illumination system |
| US9457712B2 (en) | 2013-11-21 | 2016-10-04 | Ford Global Technologies, Llc | Vehicle sun visor providing luminescent lighting |
| US9487128B2 (en) | 2013-11-21 | 2016-11-08 | Ford Global Technologies, Llc | Illuminating running board |
| US9487126B2 (en) | 2013-11-21 | 2016-11-08 | Ford Global Technologies, Llc | Photoluminescent puddle lamp |
| US9539940B2 (en) | 2013-11-21 | 2017-01-10 | Ford Global Technologies, Llc | Illuminated indicator |
| US9682649B2 (en) | 2013-11-21 | 2017-06-20 | Ford Global Technologies, Inc. | Photoluminescent winch apparatus |
| US10064256B2 (en) | 2013-11-21 | 2018-08-28 | Ford Global Technologies, Llc | System and method for remote activation of vehicle lighting |
| US9961745B2 (en) | 2013-11-21 | 2018-05-01 | Ford Global Technologies, Llc | Printed LED rylene dye welcome/farewell lighting |
| US9499113B2 (en) | 2013-11-21 | 2016-11-22 | Ford Global Technologies, Llc | Luminescent grille bar assembly |
| US9682651B2 (en) | 2013-11-21 | 2017-06-20 | Ford Global Technologies, Llc | Vehicle lighting system with improved substrate |
| US9849831B2 (en) | 2013-11-21 | 2017-12-26 | Ford Global Technologies, Llc | Printed LED storage compartment |
| US9764686B2 (en) | 2013-11-21 | 2017-09-19 | Ford Global Technologies, Llc | Light-producing assembly for a vehicle |
| US9493113B2 (en) | 2013-11-21 | 2016-11-15 | Ford Global Technologies, Llc | Photoluminescent cargo area illumination |
| US9821708B2 (en) | 2013-11-21 | 2017-11-21 | Ford Global Technologies, Llc | Illuminated exterior strip |
| US9694743B2 (en) | 2013-11-21 | 2017-07-04 | Ford Global Technologies, Llc | Dual purpose lighting assembly |
| US9782504B2 (en) | 2013-11-21 | 2017-10-10 | Ford Global Technologies, Inc. | Self-disinfecting surface with printed LEDs for a surface of a vehicle |
| US9613549B2 (en) | 2013-11-21 | 2017-04-04 | Ford Global Technologies, Llc | Illuminating badge for a vehicle |
| US9868387B2 (en) | 2013-11-21 | 2018-01-16 | Ford Global Technologies, Llc | Photoluminescent printed LED molding |
| US9902320B2 (en) | 2013-11-21 | 2018-02-27 | Ford Global Technologies, Llc | Photoluminescent color changing dome map lamp |
| US9487136B2 (en) | 2013-11-21 | 2016-11-08 | Ford Global Technologies, Llc | System and method to locate vehicle equipment |
| US9527438B2 (en) | 2013-11-21 | 2016-12-27 | Ford Global Technologies, Llc | Photoluminescent blind spot warning indicator |
| US9797575B2 (en) | 2013-11-21 | 2017-10-24 | Ford Global Technologies, Llc | Light-producing assembly for a vehicle |
| US9989216B2 (en) | 2013-11-21 | 2018-06-05 | Ford Global Technologies, Llc | Interior exterior moving designs |
| US9586518B2 (en) | 2013-11-21 | 2017-03-07 | Ford Global Technologies, Llc | Luminescent grille bar assembly |
| US9495040B2 (en) | 2013-11-21 | 2016-11-15 | Ford Global Technologies, Llc | Selectively visible user interface |
| US10400978B2 (en) | 2013-11-21 | 2019-09-03 | Ford Global Technologies, Llc | Photoluminescent lighting apparatus for vehicles |
| US9481297B2 (en) | 2013-11-21 | 2016-11-01 | Ford Global Technologies, Llc | Illuminated steering assembly |
| US9533613B2 (en) | 2013-11-21 | 2017-01-03 | Ford Global Technologies, Llc | Photoluminescent fuel filler door |
| US9583968B2 (en) | 2013-11-21 | 2017-02-28 | Ford Global Technologies, Llc | Photoluminescent disinfecting and charging bin |
| US9789810B2 (en) | 2013-11-21 | 2017-10-17 | Ford Global Technologies, Llc | Photoluminescent vehicle panel |
| US9463739B2 (en) | 2013-11-21 | 2016-10-11 | Ford Global Technologies, Llc | Sun visor with photoluminescent structure |
| US9440579B2 (en) | 2013-11-21 | 2016-09-13 | Ford Global Technologies, Llc | Photoluminescent step handle |
| US9463734B2 (en) | 2013-11-21 | 2016-10-11 | Ford Global Technologies, Llc | Illuminated seatbelt assembly |
| US9573516B2 (en) | 2013-11-21 | 2017-02-21 | Ford Global Technologies, Llc | Rear vehicle lighting system |
| US9598632B2 (en) | 2013-11-21 | 2017-03-21 | Ford Global Technologies, Llc | Method for depositing photoluminescent material |
| US9649877B2 (en) | 2013-11-21 | 2017-05-16 | Ford Global Technologies, Llc | Vehicle light system with illuminating wheel assembly |
| US9469244B2 (en) | 2013-11-21 | 2016-10-18 | Ford Global Technologies, Llc | Luminescent vehicle seal |
| US9464886B2 (en) | 2013-11-21 | 2016-10-11 | Ford Global Technologies, Llc | Luminescent hitch angle detection component |
| US9625115B2 (en) | 2013-11-21 | 2017-04-18 | Ford Global Technologies, Llc | Photoluminescent vehicle graphics |
| US9796304B2 (en) | 2013-11-21 | 2017-10-24 | Ford Global Technologies, Llc | Vehicle floor lighting system having a pivotable base with light-producing assembly coupled to base |
| US9464887B2 (en) | 2013-11-21 | 2016-10-11 | Ford Global Technologies, Llc | Illuminated hitch angle detection component |
| US9796325B2 (en) | 2013-11-21 | 2017-10-24 | Ford Global Technologies, Llc | Exterior light system for a vehicle |
| US9642223B2 (en) | 2013-11-22 | 2017-05-02 | Ford Global Technologies, Llc | Vehicle wheel assembly illumination lamp |
| US9283819B2 (en) | 2013-11-22 | 2016-03-15 | Ford Global Technologies, Llc | Vehicle wheel assembly external illumination lamp |
| KR20150109854A (en) * | 2014-03-21 | 2015-10-02 | 동우 화인켐 주식회사 | Photoluminescence Coating Composition and Photoluminescence Film Using the Same |
| JP5935869B2 (en) * | 2014-12-26 | 2016-06-15 | 日立化成株式会社 | Method for manufacturing spherical phosphor, method for manufacturing wavelength conversion type solar cell sealing material, and method for manufacturing solar cell module |
| JP6418945B2 (en) * | 2014-12-26 | 2018-11-07 | 住友ゴム工業株式会社 | Golf ball |
| EP3325562B1 (en) * | 2015-07-17 | 2020-06-03 | Hp Indigo B.V. | Electrostatic ink compositions |
| US10168039B2 (en) | 2015-08-10 | 2019-01-01 | Ford Global Technologies, Llc | Illuminated badge for a vehicle |
| US9663967B2 (en) | 2015-09-11 | 2017-05-30 | Ford Global Technologies, Llc | Illuminated latch system |
| EP3356884B1 (en) | 2015-09-29 | 2023-07-05 | Alliance for Sustainable Energy, LLC | Energy-harvesting chromogenic devices |
| US9463735B1 (en) | 2015-10-06 | 2016-10-11 | Ford Global Technologies, Llc | Vehicle visor assembly with illuminating check assembly |
| US9694739B2 (en) | 2015-11-10 | 2017-07-04 | Ford Global Technologies, Llc | Disinfecting handle |
| US9889791B2 (en) | 2015-12-01 | 2018-02-13 | Ford Global Technologies, Llc | Illuminated badge for a vehicle |
| US10023100B2 (en) | 2015-12-14 | 2018-07-17 | Ford Global Technologies, Llc | Illuminated trim assembly |
| US20170174124A1 (en) | 2015-12-16 | 2017-06-22 | Ford Global Technologies, Llc | Illuminated vehicle panel |
| US10457087B2 (en) * | 2015-12-17 | 2019-10-29 | Sicpa Holding Sa | Security element formed from at least two materials present in partially or fully overlapping areas, articles carrying the security element, and authentication methods |
| US9500333B1 (en) | 2015-12-18 | 2016-11-22 | Ford Global Technologies, Llc | Phosphorescent lighting assembly |
| US9855799B2 (en) | 2016-02-09 | 2018-01-02 | Ford Global Technologies, Llc | Fuel level indicator |
| US10300843B2 (en) | 2016-01-12 | 2019-05-28 | Ford Global Technologies, Llc | Vehicle illumination assembly |
| US10235911B2 (en) | 2016-01-12 | 2019-03-19 | Ford Global Technologies, Llc | Illuminating badge for a vehicle |
| US10501007B2 (en) | 2016-01-12 | 2019-12-10 | Ford Global Technologies, Llc | Fuel port illumination device |
| US10011219B2 (en) | 2016-01-18 | 2018-07-03 | Ford Global Technologies, Llc | Illuminated badge |
| US9927114B2 (en) | 2016-01-21 | 2018-03-27 | Ford Global Technologies, Llc | Illumination apparatus utilizing conductive polymers |
| US9517723B1 (en) | 2016-01-21 | 2016-12-13 | Ford Global Technologies, Llc | Illuminated tie-down cleat |
| US9586519B1 (en) | 2016-01-27 | 2017-03-07 | Ford Global Technologies, Llc | Vehicle rear illumination |
| US9623797B1 (en) | 2016-02-04 | 2017-04-18 | Ford Global Technologies, Llc | Lift gate lamp |
| US9499094B1 (en) | 2016-02-08 | 2016-11-22 | Ford Global Technologies, Llc | Retractable running board with long-persistence phosphor lighting |
| US9499093B1 (en) | 2016-02-08 | 2016-11-22 | Ford Global Technologies, Llc | Retractable running board with long-persistance phosphor lighting |
| US10189401B2 (en) | 2016-02-09 | 2019-01-29 | Ford Global Technologies, Llc | Vehicle light strip with optical element |
| MX2017001844A (en) | 2016-02-10 | 2018-08-08 | Ford Global Tech Llc | Vehicle badge. |
| US9664354B1 (en) | 2016-02-11 | 2017-05-30 | Ford Global Technologies, Llc | Illumination assembly |
| US9656598B1 (en) | 2016-02-23 | 2017-05-23 | Ford Global Technologies, Llc | Vehicle badge |
| US9751458B1 (en) | 2016-02-26 | 2017-09-05 | Ford Global Technologies, Llc | Vehicle illumination system |
| US10501025B2 (en) | 2016-03-04 | 2019-12-10 | Ford Global Technologies, Llc | Vehicle badge |
| US10118568B2 (en) | 2016-03-09 | 2018-11-06 | Ford Global Technologies, Llc | Vehicle badge having discretely illuminated portions |
| US9688189B1 (en) | 2016-03-09 | 2017-06-27 | Ford Global Technologies, Llc | Illuminated license plate |
| DE102017102905A1 (en) | 2016-03-11 | 2017-09-14 | Ford Global Technologies, Llc | Luminescent grill lamella device |
| US9688190B1 (en) | 2016-03-15 | 2017-06-27 | Ford Global Technologies, Llc | License plate illumination system |
| US9963001B2 (en) | 2016-03-24 | 2018-05-08 | Ford Global Technologies, Llc | Vehicle wheel illumination assembly using photoluminescent material |
| US10081296B2 (en) | 2016-04-06 | 2018-09-25 | Ford Global Technologies, Llc | Illuminated exterior strip with photoluminescent structure and retroreflective layer |
| US9902315B2 (en) | 2016-04-15 | 2018-02-27 | Ford Global Technologies, Llc | Photoluminescent lighting apparatus for vehicles |
| US9714749B1 (en) | 2016-05-10 | 2017-07-25 | Ford Global Technologies, Llc | Illuminated vehicle grille assembly |
| US9758088B1 (en) | 2016-05-10 | 2017-09-12 | Ford Global Technologies, Llc | Auxiliary lighting roof rack |
| US9738219B1 (en) | 2016-05-11 | 2017-08-22 | Ford Global Technologies, Llc | Illuminated vehicle trim |
| US10420189B2 (en) | 2016-05-11 | 2019-09-17 | Ford Global Technologies, Llc | Vehicle lighting assembly |
| US9688215B1 (en) | 2016-05-11 | 2017-06-27 | Ford Global Technologies, Llc | Iridescent vehicle applique |
| US10064259B2 (en) | 2016-05-11 | 2018-08-28 | Ford Global Technologies, Llc | Illuminated vehicle badge |
| US9821710B1 (en) | 2016-05-12 | 2017-11-21 | Ford Global Technologies, Llc | Lighting apparatus for vehicle decklid |
| US10631373B2 (en) | 2016-05-12 | 2020-04-21 | Ford Global Technologies, Llc | Heated windshield indicator |
| US9586527B1 (en) | 2016-05-18 | 2017-03-07 | Ford Global Technologies, Llc | Wheel well step assembly of vehicle |
| US9821717B1 (en) | 2016-05-18 | 2017-11-21 | Ford Global Technologies, Llc | Box step with release button that illuminates |
| US9896020B2 (en) | 2016-05-23 | 2018-02-20 | Ford Global Technologies, Llc | Vehicle lighting assembly |
| US9994144B2 (en) | 2016-05-23 | 2018-06-12 | Ford Global Technologies, Llc | Illuminated automotive glazings |
| US9925917B2 (en) | 2016-05-26 | 2018-03-27 | Ford Global Technologies, Llc | Concealed lighting for vehicles |
| US9937855B2 (en) | 2016-06-02 | 2018-04-10 | Ford Global Technologies, Llc | Automotive window glazings |
| US9803822B1 (en) | 2016-06-03 | 2017-10-31 | Ford Global Technologies, Llc | Vehicle illumination assembly |
| US10343622B2 (en) | 2016-06-09 | 2019-07-09 | Ford Global Technologies, Llc | Interior and exterior iridescent vehicle appliques |
| US10205338B2 (en) | 2016-06-13 | 2019-02-12 | Ford Global Technologies, Llc | Illuminated vehicle charging assembly |
| US9604567B1 (en) | 2016-06-15 | 2017-03-28 | Ford Global Technologies, Llc | Luminescent trailer hitch plug |
| US10131237B2 (en) | 2016-06-22 | 2018-11-20 | Ford Global Technologies, Llc | Illuminated vehicle charging system |
| US9855888B1 (en) | 2016-06-29 | 2018-01-02 | Ford Global Technologies, Llc | Photoluminescent vehicle appliques |
| US9840191B1 (en) | 2016-07-12 | 2017-12-12 | Ford Global Technologies, Llc | Vehicle lamp assembly |
| US9855797B1 (en) | 2016-07-13 | 2018-01-02 | Ford Global Technologies, Llc | Illuminated system for a vehicle |
| US9889801B2 (en) | 2016-07-14 | 2018-02-13 | Ford Global Technologies, Llc | Vehicle lighting assembly |
| US9573518B1 (en) | 2016-07-15 | 2017-02-21 | Ford Global Technologies, Llc | Floor console IR bin light |
| US9840193B1 (en) | 2016-07-15 | 2017-12-12 | Ford Global Technologies, Llc | Vehicle lighting assembly |
| US9604569B1 (en) | 2016-07-19 | 2017-03-28 | Ford Global Technologies, Llc | Window lighting system of a vehicle |
| GB201613415D0 (en) * | 2016-08-03 | 2016-09-14 | Saf-T-Glo Ltd | Markers |
| US9587967B1 (en) | 2016-08-04 | 2017-03-07 | Ford Global Technologies, Llc | Vehicle container illumination |
| US9573519B1 (en) | 2016-08-08 | 2017-02-21 | Ford Global Technologies, Llc | Engine compartment lighting to moving parts |
| US9845047B1 (en) | 2016-08-08 | 2017-12-19 | Ford Global Technologies, Llc | Light system |
| US9573520B1 (en) | 2016-08-09 | 2017-02-21 | Ford Global Technologies, Llc | Luminescent console storage bin |
| US9827903B1 (en) | 2016-08-18 | 2017-11-28 | Ford Global Technologies, Llc | Illuminated trim panel |
| US9616823B1 (en) | 2016-08-22 | 2017-04-11 | Ford Global Technologies, Llc | Illuminated badge for a vehicle |
| US10173604B2 (en) | 2016-08-24 | 2019-01-08 | Ford Global Technologies, Llc | Illuminated vehicle console |
| US10047659B2 (en) | 2016-08-31 | 2018-08-14 | Ford Global Technologies, Llc | Photoluminescent engine indicium |
| US10047911B2 (en) | 2016-08-31 | 2018-08-14 | Ford Global Technologies, Llc | Photoluminescent emission system |
| US9604568B1 (en) | 2016-09-01 | 2017-03-28 | Ford Global Technologies, Llc | Vehicle light system |
| US10308175B2 (en) | 2016-09-08 | 2019-06-04 | Ford Global Technologies, Llc | Illumination apparatus for vehicle accessory |
| US10075013B2 (en) | 2016-09-08 | 2018-09-11 | Ford Global Technologies, Llc | Vehicle apparatus for charging photoluminescent utilities |
| US10065555B2 (en) | 2016-09-08 | 2018-09-04 | Ford Global Technologies, Llc | Directional approach lighting |
| US10043396B2 (en) | 2016-09-13 | 2018-08-07 | Ford Global Technologies, Llc | Passenger pickup system and method using autonomous shuttle vehicle |
| US9593820B1 (en) | 2016-09-28 | 2017-03-14 | Ford Global Technologies, Llc | Vehicle illumination system |
| US20180086255A1 (en) | 2016-09-28 | 2018-03-29 | Ford Global Technologies, Llc | Vehicle illuminated trim |
| US9863171B1 (en) | 2016-09-28 | 2018-01-09 | Ford Global Technologies, Llc | Vehicle compartment |
| US10046688B2 (en) | 2016-10-06 | 2018-08-14 | Ford Global Technologies, Llc | Vehicle containing sales bins |
| US10137829B2 (en) | 2016-10-06 | 2018-11-27 | Ford Global Technologies, Llc | Smart drop off lighting system |
| US9707887B1 (en) | 2016-10-19 | 2017-07-18 | Ford Global Technologies, Llc | Vehicle mirror assembly |
| US9914390B1 (en) | 2016-10-19 | 2018-03-13 | Ford Global Technologies, Llc | Vehicle shade assembly |
| US10086700B2 (en) | 2016-10-20 | 2018-10-02 | Ford Global Technologies, Llc | Illuminated switch |
| US9802534B1 (en) | 2016-10-21 | 2017-10-31 | Ford Global Technologies, Llc | Illuminated vehicle compartment |
| US20180118101A1 (en) | 2016-10-28 | 2018-05-03 | Ford Global Technologies, Llc | Vehicle illuminated trim |
| US10035473B2 (en) | 2016-11-04 | 2018-07-31 | Ford Global Technologies, Llc | Vehicle trim components |
| US9902314B1 (en) | 2016-11-17 | 2018-02-27 | Ford Global Technologies, Llc | Vehicle light system |
| US9994089B1 (en) | 2016-11-29 | 2018-06-12 | Ford Global Technologies, Llc | Vehicle curtain |
| US10220784B2 (en) | 2016-11-29 | 2019-03-05 | Ford Global Technologies, Llc | Luminescent windshield display |
| US10118538B2 (en) | 2016-12-07 | 2018-11-06 | Ford Global Technologies, Llc | Illuminated rack |
| US10106074B2 (en) | 2016-12-07 | 2018-10-23 | Ford Global Technologies, Llc | Vehicle lamp system |
| US10422501B2 (en) | 2016-12-14 | 2019-09-24 | Ford Global Technologies, Llc | Vehicle lighting assembly |
| US10144365B2 (en) | 2017-01-10 | 2018-12-04 | Ford Global Technologies, Llc | Vehicle badge |
| US9815402B1 (en) | 2017-01-16 | 2017-11-14 | Ford Global Technologies, Llc | Tailgate and cargo box illumination |
| US10173582B2 (en) | 2017-01-26 | 2019-01-08 | Ford Global Technologies, Llc | Light system |
| US10053006B1 (en) | 2017-01-31 | 2018-08-21 | Ford Global Technologies, Llc | Illuminated assembly |
| US9849830B1 (en) | 2017-02-01 | 2017-12-26 | Ford Global Technologies, Llc | Tailgate illumination |
| US9896023B1 (en) | 2017-02-09 | 2018-02-20 | Ford Global Technologies, Llc | Vehicle rear lighting assembly |
| US10427593B2 (en) | 2017-02-09 | 2019-10-01 | Ford Global Technologies, Llc | Vehicle light assembly |
| US10844658B2 (en) | 2017-02-27 | 2020-11-24 | Alliance For Sustainable Energy, Llc | Energy-harvesting chromogenic devices |
| DE202017101205U1 (en) | 2017-03-02 | 2017-03-21 | Ford Global Technologies, Llc | Illumination system for a license plate |
| US9849829B1 (en) | 2017-03-02 | 2017-12-26 | Ford Global Technologies, Llc | Vehicle light system |
| US9758090B1 (en) | 2017-03-03 | 2017-09-12 | Ford Global Technologies, Llc | Interior side marker |
| US10240737B2 (en) | 2017-03-06 | 2019-03-26 | Ford Global Technologies, Llc | Vehicle light assembly |
| US10195985B2 (en) | 2017-03-08 | 2019-02-05 | Ford Global Technologies, Llc | Vehicle light system |
| US10399483B2 (en) | 2017-03-08 | 2019-09-03 | Ford Global Technologies, Llc | Vehicle illumination assembly |
| US10150396B2 (en) | 2017-03-08 | 2018-12-11 | Ford Global Technologies, Llc | Vehicle cup holder assembly with photoluminescent accessory for increasing the number of available cup holders |
| US10611298B2 (en) | 2017-03-13 | 2020-04-07 | Ford Global Technologies, Llc | Illuminated cargo carrier |
| US10166913B2 (en) | 2017-03-15 | 2019-01-01 | Ford Global Technologies, Llc | Side marker illumination |
| US10465879B2 (en) | 2017-03-27 | 2019-11-05 | Ford Global Technologies, Llc | Vehicular light assemblies with LED-excited photoluminescent lightguide |
| US10483678B2 (en) | 2017-03-29 | 2019-11-19 | Ford Global Technologies, Llc | Vehicle electrical connector |
| US10569696B2 (en) | 2017-04-03 | 2020-02-25 | Ford Global Technologies, Llc | Vehicle illuminated airflow control device |
| US10023110B1 (en) | 2017-04-21 | 2018-07-17 | Ford Global Technologies, Llc | Vehicle badge sensor assembly |
| US10035463B1 (en) | 2017-05-10 | 2018-07-31 | Ford Global Technologies, Llc | Door retention system |
| US10399486B2 (en) | 2017-05-10 | 2019-09-03 | Ford Global Technologies, Llc | Vehicle door removal and storage |
| US20180326899A1 (en) | 2017-05-10 | 2018-11-15 | Ford Global Technologies, Llc | Illuminated hinge assembly |
| US11043335B2 (en) | 2017-05-10 | 2021-06-22 | Alliance For Sustainable Energy, Llc | Multilayer carbon nanotube film-containing devices |
| US9963066B1 (en) | 2017-05-15 | 2018-05-08 | Ford Global Technologies, Llc | Vehicle running board that provides light excitation |
| US20180336786A1 (en) | 2017-05-19 | 2018-11-22 | Ford Global Technologies, Llc | Collision avoidance method and system |
| US10871405B2 (en) * | 2017-05-30 | 2020-12-22 | The Boeing Company | Indicator device and method |
| US10059238B1 (en) | 2017-05-30 | 2018-08-28 | Ford Global Technologies, Llc | Vehicle seating assembly |
| US10144337B1 (en) | 2017-06-02 | 2018-12-04 | Ford Global Technologies, Llc | Vehicle light assembly |
| US10493904B2 (en) | 2017-07-17 | 2019-12-03 | Ford Global Technologies, Llc | Vehicle light assembly |
| US10502690B2 (en) | 2017-07-18 | 2019-12-10 | Ford Global Technologies, Llc | Indicator system for vehicle wear components |
| US10137831B1 (en) | 2017-07-19 | 2018-11-27 | Ford Global Technologies, Llc | Vehicle seal assembly |
| US10160405B1 (en) | 2017-08-22 | 2018-12-25 | Ford Global Technologies, Llc | Vehicle decal assembly |
| US10186177B1 (en) | 2017-09-13 | 2019-01-22 | Ford Global Technologies, Llc | Vehicle windshield lighting assembly |
| US10137825B1 (en) | 2017-10-02 | 2018-11-27 | Ford Global Technologies, Llc | Vehicle lamp assembly |
| US10391943B2 (en) | 2017-10-09 | 2019-08-27 | Ford Global Technologies, Llc | Vehicle lamp assembly |
| US10207636B1 (en) | 2017-10-18 | 2019-02-19 | Ford Global Technologies, Llc | Seatbelt stowage assembly |
| US10179542B1 (en) | 2017-10-19 | 2019-01-15 | Ford Global Technologies, Llc | Vehicle climate status indicator |
| US10189414B1 (en) | 2017-10-26 | 2019-01-29 | Ford Global Technologies, Llc | Vehicle storage assembly |
| CN108070306B (en) * | 2017-12-20 | 2020-04-21 | 成都斯特斯科技有限公司 | Laser stealth coating and preparation method thereof |
| US10723258B2 (en) | 2018-01-04 | 2020-07-28 | Ford Global Technologies, Llc | Vehicle lamp assembly |
| US10723257B2 (en) | 2018-02-14 | 2020-07-28 | Ford Global Technologies, Llc | Multi-color luminescent grille for a vehicle |
| US10627092B2 (en) | 2018-03-05 | 2020-04-21 | Ford Global Technologies, Llc | Vehicle grille assembly |
| US10281113B1 (en) | 2018-03-05 | 2019-05-07 | Ford Global Technologies, Llc | Vehicle grille |
| US10457196B1 (en) | 2018-04-11 | 2019-10-29 | Ford Global Technologies, Llc | Vehicle light assembly |
| US10703263B2 (en) | 2018-04-11 | 2020-07-07 | Ford Global Technologies, Llc | Vehicle light system |
| US10778223B2 (en) | 2018-04-23 | 2020-09-15 | Ford Global Technologies, Llc | Hidden switch assembly |
| CA3004436C (en) | 2018-05-09 | 2021-06-01 | Paige Whitehead | Biodegradable light wand |
| US10576893B1 (en) | 2018-10-08 | 2020-03-03 | Ford Global Technologies, Llc | Vehicle light assembly |
| US10720551B1 (en) | 2019-01-03 | 2020-07-21 | Ford Global Technologies, Llc | Vehicle lamps |
| US10576879B1 (en) | 2019-02-14 | 2020-03-03 | Ford Global Technologies, Llc | Retractable illuminated running board |
| CN110938403B (en) * | 2019-12-02 | 2021-08-17 | 武汉市科达云石护理材料有限公司 | Fluorescent anti-skid adhesive and preparation method thereof |
| NL2028298B1 (en) * | 2021-05-26 | 2022-12-08 | Renewaball B V | Recyclable product and method of recycling a mixed waste stream comprising said product |
Citations (98)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2787558A (en) * | 1955-01-27 | 1957-04-02 | Firth Carpet Company Inc | Process of producing phosphorescent yarn |
| US3022189A (en) * | 1961-01-19 | 1962-02-20 | Du Pont | Daylight fluorescent coated fabric |
| US3508810A (en) * | 1967-07-19 | 1970-04-28 | Vari Light Corp | Photochromic systems |
| US3560211A (en) * | 1967-09-22 | 1971-02-02 | Horizons Research Inc | Light sensitive leuco dye systems containing no molecular oxygen therein |
| US3562172A (en) * | 1968-10-28 | 1971-02-09 | Fuji Photo Film Co Ltd | Photochromic compound and composition containing the same |
| US3578602A (en) * | 1967-08-30 | 1971-05-11 | Fuji Photo Film Co Ltd | Photochromic compound |
| US3650812A (en) * | 1969-12-24 | 1972-03-21 | Ford Motor Co | Acrylic-siloxane resin paint and painted article |
| US3654190A (en) * | 1970-05-28 | 1972-04-04 | Us Navy | Fire retardant intumescent paint |
| US3666352A (en) * | 1970-01-22 | 1972-05-30 | Charles A Wagner | Rate controlled photochromic lenses of vinyl chloride-vinyl acetate copolymer containing a mercury thiocarbazone compound |
| US3668189A (en) * | 1970-08-24 | 1972-06-06 | Allied Chem | Fluorescent polycarbonamides |
| US3714181A (en) * | 1970-12-31 | 1973-01-30 | American Cyanamid Co | 2-aryl-5,10-diphenylphenanthro(9,10-d)azoles |
| US3738299A (en) * | 1972-06-22 | 1973-06-12 | M Packler | Emblems which will glow in the dark and the method of making them |
| US3873390A (en) * | 1972-09-27 | 1975-03-25 | Richard K Cornell | Phosphorescent, fluorescent and reflective coated sheets or films and compositions and method for making the same |
| US3884697A (en) * | 1969-03-31 | 1975-05-20 | Eiichi Inoue | Photographic process utilizing spiropyran compound dispersed in nitrocellulose films with high nitrogen content |
| US3936970A (en) * | 1971-05-10 | 1976-02-10 | Hodges John A | Fishing lure and method of fishing |
| US3957678A (en) * | 1973-01-11 | 1976-05-18 | U.S. Philips Corporation | Method of manufacturing a luminescent sulfide |
| US4025661A (en) * | 1972-11-13 | 1977-05-24 | Rca Corporation | Method of making viewing-screen structure for a cathode-ray tube |
| US4028118A (en) * | 1972-05-30 | 1977-06-07 | Pilot Ink Co., Ltd. | Thermochromic materials |
| US4188449A (en) * | 1977-08-04 | 1980-02-12 | Eastman Kodak Company | Phosphorescent screens |
| US4208300A (en) * | 1973-07-11 | 1980-06-17 | Gravisse Philippe E | Photoluminescent materials and method of manufacturing same |
| US4268134A (en) * | 1979-03-07 | 1981-05-19 | Corning Glass Works | Lightweight laminated photochromic lenses |
| US4375373A (en) * | 1978-12-29 | 1983-03-01 | Toro Ganryo Kogyo Co., Ltd. | Method of coating inorganic pigments (ultramarine and bronze powder) with dense amorphous silica |
| US4379100A (en) * | 1981-02-02 | 1983-04-05 | Ex-Cell-O Corporation | Polyurethane molding process with siloxane internal release agent |
| US4425377A (en) * | 1981-07-22 | 1984-01-10 | Rca Corporation | Method of making a cathode-ray tube having a conductive internal coating exhibiting reduced arcing current |
| US4425161A (en) * | 1980-11-27 | 1984-01-10 | Yutaka Shibahashi | Thermochromic materials |
| US4440672A (en) * | 1982-03-22 | 1984-04-03 | American Optical Corporation | Photochromic composition resistant to fatigue |
| US4451504A (en) * | 1983-05-20 | 1984-05-29 | North American Philips Consumer Electronics Corp. | Process for applying phosphor to the aperture mask of a cathode ray tube |
| US4567019A (en) * | 1977-05-11 | 1986-01-28 | Graphic Controls Corporation | Color reversing compositions |
| US4637698A (en) * | 1983-11-04 | 1987-01-20 | Ppg Industries, Inc. | Photochromic compound and articles containing the same |
| US4663214A (en) * | 1985-01-04 | 1987-05-05 | Coburn Jr Joseph W | Phosphorescent material and process of manufacture |
| US4717770A (en) * | 1986-02-27 | 1988-01-05 | Sumitomo Chemical Co., Ltd. | Process for producing epsilon-caprolactam |
| US4717710A (en) * | 1985-01-17 | 1988-01-05 | Matsui Shikiso Chemical Co. Ltd. | Thermochromic composition |
| US4720356A (en) * | 1982-03-22 | 1988-01-19 | American Optical Corporation | Photochromic composition resistant to fatigue |
| US4729907A (en) * | 1987-02-24 | 1988-03-08 | Rca Corporation | Method of making a viewing screen structure for a cathode-ray tube |
| US4734295A (en) * | 1985-01-07 | 1988-03-29 | Liu P Dong Guang | Glare control |
| US4818096A (en) * | 1986-06-17 | 1989-04-04 | The Plessey Company Plc | Photoreactive lenses with adamantane spiro compounds |
| US4826550A (en) * | 1985-11-28 | 1989-05-02 | Matui Shikiso Chemical Co., Ltd. | Process for preparing molded product of thermochromic polyvinyl chloride |
| US4826977A (en) * | 1986-05-15 | 1989-05-02 | The Plessey Company Plc | Photochromic spiropyran compounds |
| US4830875A (en) * | 1985-10-10 | 1989-05-16 | Quantex Corporation | Photoluminescent materials and associated process and infrared sensing device |
| US4898895A (en) * | 1986-12-30 | 1990-02-06 | Nippon Oil And Fats Co., Ltd. | Antifouling pain having a polyacrylate component with pendent silyl or siloxane groups |
| US4910252A (en) * | 1986-07-07 | 1990-03-20 | Kansai Paint Co., Ltd. | Siloxane polymer antifouling paint composition containing polysiloxanes |
| US4913544A (en) * | 1986-05-01 | 1990-04-03 | Pilkington Plc | Photochromic articles |
| US4921727A (en) * | 1988-12-21 | 1990-05-01 | Rca Licensing Corporation | Surface treatment of silica-coated phosphor particles and method for a CRT screen |
| US4927180A (en) * | 1986-08-22 | 1990-05-22 | Plessey Overseas Limited | Marking of articles with photochromic compounds |
| US5007647A (en) * | 1989-12-15 | 1991-04-16 | Sports Glow, Inc. | Golf ball and method of making same |
| US5023015A (en) * | 1989-12-19 | 1991-06-11 | Gte Products Corporation | Method of phosphor preparation |
| US5176905A (en) * | 1989-11-30 | 1993-01-05 | Shiseido Co., Ltd. | Photochromic flesh-colored pigment and process for producing the same |
| US5185390A (en) * | 1990-03-07 | 1993-02-09 | Ppg Industries, Inc. | Water strippable photochromic resin composition |
| US5219625A (en) * | 1991-07-09 | 1993-06-15 | The Pilot Ink Co., Ltd. | Thermochromic laminate member and toy utilizing the same |
| US5221288A (en) * | 1990-10-09 | 1993-06-22 | Matsui Shikiso Chemical Co., Ltd. | Thermochromic dyeing method and cellulose product dyed thereby |
| US5223330A (en) * | 1990-11-28 | 1993-06-29 | Precision Fabrics Group, Inc. | Phosphorescent fiber reinforced plastic article and process for making the same |
| US5292549A (en) * | 1992-10-23 | 1994-03-08 | Armco Inc. | Metallic coated steel having a siloxane film providing temporary corrosion protection and method therefor |
| US5294375A (en) * | 1991-08-20 | 1994-03-15 | Polaroid Corporation | Thermochromic materials |
| US5321069A (en) * | 1992-11-25 | 1994-06-14 | Afterglow Accent Yarns, Inc. | Process for producing phosphorescent yarn and yarn produced by the process |
| US5378897A (en) * | 1992-12-09 | 1995-01-03 | Fuji Photo Film Co., Ltd. | Radiation image storage panel |
| US5387458A (en) * | 1990-12-06 | 1995-02-07 | Minnesota Mining And Manufacturing Company | Articles exhibiting durable fluorescence with an ultraviolet screening layer |
| US5389093A (en) * | 1992-04-01 | 1995-02-14 | Howell; Wesley A. | Wetness indicating diaper |
| US5391327A (en) * | 1992-09-25 | 1995-02-21 | Transitions Optical, Inc. | Photochromic compositions of improved fatigue resistance |
| US5395673A (en) * | 1992-04-23 | 1995-03-07 | Hunt; Gary B. | Non-slip surface |
| US5424006A (en) * | 1993-04-28 | 1995-06-13 | Nemoto & Co., Ltd. | Phosphorescent phosphor |
| US5427415A (en) * | 1992-12-09 | 1995-06-27 | Wallace Computer Services, Inc. | Heat sensitive system and use thereof |
| US5480482A (en) * | 1991-11-04 | 1996-01-02 | The United States Of America As Represented By The Secretary Of The Navy | Reversible thermochromic pigments |
| US5490344A (en) * | 1994-03-22 | 1996-02-13 | Bussiere; Robert A. | Glow-in-the-dark material for fishing accessories |
| US5605734A (en) * | 1989-11-02 | 1997-02-25 | Basf Corporation | Phosphorescent directional signals and manufacturing method |
| US5607621A (en) * | 1994-12-28 | 1997-03-04 | Ykk Corporation | Phosphorescent synthetic resin material method for production thereof, and formed article |
| US5618063A (en) * | 1992-12-09 | 1997-04-08 | Wallace Computer Services, Inc. | Multicolor heat-sensitive verification and highlighting system |
| US5630869A (en) * | 1988-01-12 | 1997-05-20 | Sicipa Holding S.A. | Reversibly photochromic printing inks |
| US5708184A (en) * | 1995-07-31 | 1998-01-13 | Bayer Aktiengesellschaft | Process for the preparation of substituted aminocarbonyltriazolinones |
| US5716723A (en) * | 1996-03-07 | 1998-02-10 | Van Cleef; James Gresham | Glow in the dark shoe sole |
| US5717282A (en) * | 1995-02-20 | 1998-02-10 | U.S. Philips Corporation | Display device comprising a display screen having a light-absorbing coating |
| US5728758A (en) * | 1993-12-13 | 1998-03-17 | Transitions Optical, Inc. | Coating composition and articles having a cured coating |
| US5731658A (en) * | 1994-11-30 | 1998-03-24 | Honeywell Inc. | Ultraviolet binder for phosphor fluorescent light box |
| US5730961A (en) * | 1997-01-24 | 1998-03-24 | Goudjil; Kamal | Metamorphic nail polish |
| US5744233A (en) * | 1994-09-09 | 1998-04-28 | U.S. Philips Corporation | Method of coating luminescent powders, luminescent powders and coated object |
| US5753146A (en) * | 1996-03-29 | 1998-05-19 | Transitions Optical, Inc. | Photochromic naphthopyran compositions of neutral color |
| US5770115A (en) * | 1996-04-19 | 1998-06-23 | Ppg Industries, Inc. | Photochromic naphthopyran compositions of improved fatigue resistance |
| US6027810A (en) * | 1994-10-07 | 2000-02-22 | Minnesota Mining & Manufacturing | Radiographic intensifying screen with antistat |
| US6046455A (en) * | 1998-01-30 | 2000-04-04 | Segan Industries | Integrating ultraviolet exposure detection devices |
| US6048347A (en) * | 1995-11-01 | 2000-04-11 | Micro Medical Devices, Inc. | Lens storage and folding apparatus |
| US6060428A (en) * | 1992-12-09 | 2000-05-09 | Wallace Computer Services, Inc. | Heat-sensitive chromogenic system |
| US6177487B1 (en) * | 1995-11-03 | 2001-01-23 | Basf Coatings Ag | Aqueous powder paint dispersions |
| US6196241B1 (en) * | 1999-05-19 | 2001-03-06 | Denise Doolan | Color changing umbrella |
| US6201057B1 (en) * | 1998-02-23 | 2001-03-13 | Therma-Tru Corporation | Weatherable coating and stain system for thermoset or thermoplastic composite surfaces |
| US6207077B1 (en) * | 2000-02-18 | 2001-03-27 | Orion 21 A.D. Pty Ltd | Luminescent gel coats and moldable resins |
| US6344233B1 (en) * | 1996-10-15 | 2002-02-05 | Institute For Radiological Image Sciences, Inc. | Method of producing a phosphor screen |
| US6358160B1 (en) * | 1997-10-03 | 2002-03-19 | Performance Dynamics Llc | Golf ball with water immersion indicator |
| US6359048B1 (en) * | 1999-06-05 | 2002-03-19 | Van Duynhoven Debra May | Tintable luminescent paint |
| US6375864B1 (en) * | 1998-11-10 | 2002-04-23 | M.A. Hannacolor, A Division Of M.A. Hanna Company | Daylight/nightglow colored phosphorescent plastic compositions and articles |
| US6391492B1 (en) * | 1992-04-05 | 2002-05-21 | Canon Kabushiki Kaisha | Secondary battery |
| US6508732B1 (en) * | 2000-07-03 | 2003-01-21 | Mildred Kinghorn Romberger | Tennis ball |
| US6514594B1 (en) * | 2000-11-09 | 2003-02-04 | Avery Dennison Corporation | Fluorescent polymeric articles having screening layer formed from U.V. light absorbing polymer |
| US6553696B1 (en) * | 1999-09-17 | 2003-04-29 | Robert Foster, Sr. | Flourescent drink rim |
| US20040009833A1 (en) * | 2002-07-10 | 2004-01-15 | Ja-Ru, Inc. | Glow-in-the-dark wrist toy |
| US6710127B2 (en) * | 2000-09-29 | 2004-03-23 | Byk- Chemie Gmbh | Levelling agents for surface coatings |
| US6861467B2 (en) * | 2002-06-28 | 2005-03-01 | Okitsumo Incorporated | Powder coating composition |
| US6870024B2 (en) * | 2002-09-09 | 2005-03-22 | Byk-Chemie Gmbh | Polymeric urea-urethane rheology control agents and a process for their preparation |
| US6894124B2 (en) * | 2000-11-01 | 2005-05-17 | Kansai Paint Co., Ltd. | High solid paint compositions |
| US7033712B2 (en) * | 2003-01-30 | 2006-04-25 | Thomson Licensing | Method of manufacturing a color filter cathode ray tube (CRT) |
Family Cites Families (185)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2522704A (en) | 1939-12-08 | 1950-09-19 | Laval Jacques Hjaimar De | Method and apparatus to treat material in form of pieces or powder with gases |
| US2527365A (en) | 1945-05-22 | 1950-10-24 | Rca Corp | Doubly activated infrared phosphors |
| US3212898A (en) | 1962-11-21 | 1965-10-19 | American Cyanamid Co | Photosensitive compositions of matter comprising photochromic materials suspended in polyester binders |
| US3522143A (en) | 1966-08-18 | 1970-07-28 | Libbey Owens Ford Co | Phototropic units |
| US3595804A (en) * | 1968-10-30 | 1971-07-27 | Rca Corp | Method for preparing zinc and zinccadmium sulfide phosphors |
| US3627690A (en) | 1969-10-01 | 1971-12-14 | Itek Corp | Photochromic naphthopyran compositions |
| US3912677A (en) | 1970-05-14 | 1975-10-14 | Ici Australia Ltd | Compounds |
| JPS551195B2 (en) | 1972-09-27 | 1980-01-12 | ||
| US4210953A (en) | 1973-12-13 | 1980-07-01 | Stone Wilfred S | Self-illuminated case |
| DE2515474A1 (en) | 1974-04-11 | 1975-10-30 | Raychem Corp | THERMOCHROME PAINTS |
| US3980602A (en) * | 1975-02-28 | 1976-09-14 | E. I. Du Pont De Nemours And Company | Acrylic polymer dispersant for aqueous acrylic coating compositions |
| US4121011A (en) | 1975-11-28 | 1978-10-17 | Raychem Corporation | Polymeric article coated with a thermochromic paint |
| BE852915A (en) * | 1977-03-25 | 1977-09-26 | Bric Bureau De Rech Pour L Inn | COATED PHOTOLUMINESCENT TEXTILES |
| US4130760A (en) | 1977-06-29 | 1978-12-19 | Minnesota Mining And Manufacturing Company | Reusable radiation monitor |
| US4362799A (en) | 1978-04-28 | 1982-12-07 | Canon Kabushiki Kaisha | Image-holding member with a curable epoxyacrylate resin insulating layer |
| US4342668A (en) | 1978-09-08 | 1982-08-03 | American Optical Corporation | Photochromic compounds |
| US4215010A (en) | 1978-09-08 | 1980-07-29 | American Optical Corporation | Photochromic compounds |
| JPS5937037B2 (en) | 1978-11-09 | 1984-09-07 | 株式会社東芝 | Method for manufacturing phosphor |
| US4286957A (en) | 1979-01-10 | 1981-09-01 | Essilor International "Cie Generale D'optique" | Process of integrating a photochromic substance into an ophthalmic lens and a photochromic lens of organic material |
| US4304833A (en) | 1979-12-26 | 1981-12-08 | Polaroid Corporation | Photographic products and processes employing triarylmethane compounds |
| US4289497A (en) | 1980-09-02 | 1981-09-15 | American Optical Corporation | Gradient photochromic lens and method selectively reducing photochromic activity |
| JPS57167380A (en) | 1981-04-08 | 1982-10-15 | Pilot Ink Co Ltd | Thermochromic material |
| JPS59155800A (en) | 1983-02-24 | 1984-09-04 | 富士写真フイルム株式会社 | Storable fluorescent sheet |
| JPS6032234A (en) | 1983-08-03 | 1985-02-19 | Hitachi Ltd | Formation of filming film of cathode-ray tube |
| US4699473A (en) | 1983-08-08 | 1987-10-13 | American Optical Corporation | Trifluoromethyl substituted spirooxazine photochromic dyes |
| US4623579A (en) * | 1983-10-04 | 1986-11-18 | Multi-Tex Products Corp. | Yarn product with combined fluorescent-phosphorescent appearance and method |
| GB8402801D0 (en) | 1984-02-02 | 1984-03-07 | Ici Plc | Dispersion |
| JPH0753665B2 (en) | 1984-04-20 | 1995-06-07 | 財団法人癌研究会 | Anti-metastatic agent |
| US4857228A (en) | 1984-04-24 | 1989-08-15 | Sunstone Inc. | Phosphors and methods of preparing the same |
| US4602263A (en) | 1984-09-04 | 1986-07-22 | Polaroid Corporation | Thermal imaging method |
| CA1240883A (en) | 1985-01-30 | 1988-08-23 | Norikazu Nakasuji | Thermochromic textile material |
| US4640797A (en) * | 1985-06-11 | 1987-02-03 | Jones And Vining, Incorporated | Phosphorescent polymer-containing compositions and articles made therefrom |
| US4629583A (en) | 1985-06-11 | 1986-12-16 | Jones And Vining, Incorporated | Phosphorescent polymer-containing compositions and articles made therefrom |
| US4880667A (en) | 1985-09-24 | 1989-11-14 | Ppg Industries, Inc. | Photochromic plastic article and method for preparing same |
| US4695336A (en) | 1985-10-11 | 1987-09-22 | Coburn Jr Joseph W | Phosphorescent material and process of manufacture |
| US4884860A (en) | 1986-02-05 | 1989-12-05 | Brown David C | Linear lens and method for concentrating radiant energy and multiplying phosphor luminance output intensity |
| US4698296A (en) | 1986-03-14 | 1987-10-06 | Gaf Corporation | Processless color imaging and film therefor |
| US4835475A (en) * | 1986-11-17 | 1989-05-30 | Niichi Hanakura | Battery tester including a thermochromic material |
| NL8702089A (en) | 1987-09-04 | 1989-04-03 | Efka Chemicals Bv | DISPENSANT. |
| ATE100006T1 (en) | 1987-04-09 | 1994-01-15 | Ceramics Process Systems | MANUFACTURE OF COMPLEX CERAMIC AND METALLIC HIGH-PERFORMANCE MOLDS. |
| US4781647A (en) | 1987-05-04 | 1988-11-01 | Hasbro, Inc. | Toy doll construction with phosphorescent hair fibers |
| US4759453A (en) | 1987-06-26 | 1988-07-26 | Paetzold James M | Luminescent baby bottle |
| US4942213A (en) | 1987-12-04 | 1990-07-17 | Byk-Chemie Gmbh | Addition compounds useful as dispersing agents and dispersion stabilizers, process for producing them, their use and solids coated therewith |
| US5807625A (en) | 1988-01-12 | 1998-09-15 | Sicpa Holding S.A. | Security document with reversibly photochromic printing inks |
| DE68916070T2 (en) | 1988-03-16 | 1994-10-13 | Mitsubishi Rayon Co | Phosphorus paste compositions and coatings obtained therewith. |
| JPH01249436A (en) | 1988-03-31 | 1989-10-04 | Toray Ind Inc | Transparent conductive film and its manufacture |
| US5435994A (en) | 1988-08-23 | 1995-07-25 | Ultraset Limited Partnership | Quick-drying nail coating method and composition |
| US4943896A (en) | 1988-11-21 | 1990-07-24 | Tony Johnson | Production of improved infant care articles |
| US5081171A (en) * | 1989-02-14 | 1992-01-14 | Nixon Charles R | Composition for sealing of painted or metal surfaces |
| DE3930687A1 (en) | 1989-09-14 | 1991-04-11 | Byk Chemie Gmbh | Phosphoric acid esters, process for their preparation and their use as dispersing agents |
| JPH03143180A (en) | 1989-10-30 | 1991-06-18 | Pioneer Electron Corp | Organic fluorescent screen |
| CA2032992C (en) | 1989-12-29 | 2001-04-10 | Peter H. Quednau | Dispersing agents, their use and solids coated therewith |
| US5066818A (en) | 1990-03-07 | 1991-11-19 | Ppg Industries, Inc. | Photochromic naphthopyran compounds |
| JPH03261596A (en) | 1990-03-10 | 1991-11-21 | Dainippon Printing Co Ltd | Card and method for identifying card |
| US5260252A (en) | 1990-07-24 | 1993-11-09 | Nashua Corporation | Thermal latent image material and method of producing and developing the same |
| US5164127A (en) * | 1990-10-02 | 1992-11-17 | Cook Composites And Polymers Co. | Method of preparing molded coatings for gel coated composites |
| US5149568A (en) | 1990-11-19 | 1992-09-22 | Beck Michael P | Glow in the dark artwork |
| US5135591A (en) | 1990-11-28 | 1992-08-04 | Precision Fabrics Group, Inc. | Process of making a phosphorescent fiber reinforced plastic article |
| US5409797A (en) | 1991-03-04 | 1995-04-25 | Fuji Photo Film Co., Ltd. | Heat-sensitive recording material for laser recording |
| US6312782B1 (en) | 1991-03-18 | 2001-11-06 | Rochelle L. Goldberg | Discreet shaped colored polymeric objects in a transparent or translucent matrix |
| US5352649A (en) | 1991-07-04 | 1994-10-04 | The Pilot Ink Co., Ltd. | Thermochromic laminate member, and composition and sheet for producing the same |
| JP2618596B2 (en) | 1991-07-08 | 1997-06-11 | ローン−プーラン・ロレ・ソシエテ・アノニム | Novel composition based on taxane derivatives |
| US5248916A (en) | 1991-10-02 | 1993-09-28 | Zenith Electronics Corporation | Chlorinated silane and alkoxysilane coatings for cathode ray tubes |
| US5132043A (en) | 1991-12-24 | 1992-07-21 | Gte Products Corporation | Method of preparing small particle size borate phosphor |
| US5916541A (en) | 1992-06-25 | 1999-06-29 | Stewart; Ernest G. | Water resistant sunscreen and insect repellent composition |
| GB9225346D0 (en) | 1992-12-03 | 1993-01-27 | Pilkington Plc | Photochromic compounds |
| US5344191A (en) | 1992-12-09 | 1994-09-06 | Wallace Computer Services, Inc. | Hidden entry system and use thereof |
| US5356149A (en) | 1992-12-23 | 1994-10-18 | Kane Patrick E | Injection molded water-soluble golf ball |
| KR960702118A (en) | 1993-04-20 | 1996-03-28 | 워렌 리챠드 보비 | PHOTOGRAPHIC ELEMENTS COMPRISING ANTISTATIC LAYERS |
| EP0646631B1 (en) * | 1993-10-05 | 2004-01-28 | Hitachi Maxell Ltd. | Light-emitting ink composition |
| US5445611A (en) | 1993-12-08 | 1995-08-29 | Non-Invasive Monitoring Company (Nimco) | Enhancement of transdermal delivery with ultrasound and chemical enhancers |
| US5997849A (en) | 1993-12-29 | 1999-12-07 | Chromatic Technologies, Inc. | Thermochromic ink formulations, nail lacquer and methods of use |
| US5708181A (en) * | 1994-03-11 | 1998-01-13 | Otsuka Kagaku Kabushiki Kaisha | Spiropyran compound |
| US5665793A (en) * | 1994-06-09 | 1997-09-09 | Anders; Irving | Phosphorescent highway paint composition |
| US6005024A (en) | 1994-06-09 | 1999-12-21 | Anders; Irving | Phosphorescent epoxy overlay |
| JP3585005B2 (en) | 1994-08-12 | 2004-11-04 | 大日本インキ化学工業株式会社 | Curable resin composition for water-based paint |
| JPH0899384A (en) * | 1994-09-30 | 1996-04-16 | Ykk Kk | Luminous material |
| DE69504862T2 (en) | 1994-11-10 | 1999-05-12 | Minnesota Mining & Mfg | PHOTOGRAPHIC ELEMENT WITH AN ANTISTATIC LAYER AND METHOD FOR PRODUCING AN ELEMENT WITH ANTISTATIC PROPERTIES |
| US6194539B1 (en) | 1994-11-22 | 2001-02-27 | Daicel Chemical Industries, Inc. | Polylactone having amino groups, a process for the preparation thereof, a compound having amino group, a composition for coatings, a composition for printing inks |
| US5692895A (en) * | 1995-01-30 | 1997-12-02 | Ormco Corporation | Luminescent orthodontic appliances |
| US5558187A (en) | 1995-06-07 | 1996-09-24 | Aberle; David H. | Brake apparatus for a rotating shaft |
| US5658500A (en) | 1995-06-14 | 1997-08-19 | Transitions Optical, Inc. | Substituted naphthopyrans |
| US5973034A (en) | 1995-10-11 | 1999-10-26 | Nippon Kayaku Kabushiki Kaisha | (Oxide or sulfide) powder epoxy (meth) acrylate w/glass and/or metal |
| US5581090A (en) | 1995-10-25 | 1996-12-03 | Solartech Enterprises, Llc | Photochromic ultraviolet detector |
| JP3595046B2 (en) | 1995-11-08 | 2004-12-02 | ビーエーエスエフディスパージョン株式会社 | Polymer dispersant, production method thereof, and emulsion polymerization method using the same |
| DE19547327C2 (en) | 1995-12-19 | 1999-08-26 | Daimler Chrysler Ag | Layer structure with a photochromic material, process for its preparation and its use |
| US5885482A (en) | 1995-12-28 | 1999-03-23 | Canon Kabushiki Kaisha | Liquid crystal device, production process thereof and liquid crystal apparatus |
| US5674437A (en) | 1996-02-28 | 1997-10-07 | Glotex Corporation | Method of providing luminescence to fibrous materials |
| US5789015A (en) | 1996-06-26 | 1998-08-04 | Innotech, Inc. | Impregnation of plastic substrates with photochromic additives |
| JPH1036834A (en) | 1996-07-16 | 1998-02-10 | Riken Vinyl Kogyo Kk | Phosphor composition |
| US5998085A (en) | 1996-07-23 | 1999-12-07 | 3M Innovative Properties | Process for preparing high resolution emissive arrays and corresponding articles |
| US5753597A (en) | 1996-08-20 | 1998-05-19 | Chevron Chemical Company | Polymeric dispersants |
| US5938554A (en) | 1996-09-30 | 1999-08-17 | Borg-Warner Automotive, Inc. | Roller chain link plate profile |
| TW445380B (en) | 1996-10-23 | 2001-07-11 | Sumitomo Chemical Co | Plasma display front panel |
| TW358895B (en) | 1996-12-26 | 1999-05-21 | Sumitomo Chemical Co | Plasma display front panel |
| US5774997A (en) | 1997-01-02 | 1998-07-07 | Performance Dynamics Llc | Golf ball out-of-round indicator |
| US6268458B1 (en) | 1997-01-07 | 2001-07-31 | Corning Precision Lens | Coupler fluids for projection televisions |
| US6197218B1 (en) | 1997-02-24 | 2001-03-06 | Superior Micropowders Llc | Photoluminescent phosphor powders, methods for making phosphor powders and devices incorporating same |
| EP1176575A1 (en) | 1997-03-17 | 2002-01-30 | Magiccom | Flexible label, roll and stack |
| TW417025B (en) | 1997-04-10 | 2001-01-01 | Sumitomo Chemical Co | Front plate for plasma display |
| US6746724B1 (en) | 1997-04-11 | 2004-06-08 | Infosight Corporation | Dual paint coat laser-marking labeling system, method, and product |
| US5989135A (en) | 1997-04-28 | 1999-11-23 | Night & Day Golf, Inc. | Luminescent golf ball |
| US6013980A (en) * | 1997-05-09 | 2000-01-11 | Advanced Refractory Technologies, Inc. | Electrically tunable low secondary electron emission diamond-like coatings and process for depositing coatings |
| US5975696A (en) | 1997-05-12 | 1999-11-02 | Kohan; George | Process for rendering plastic substrate photochromic |
| DE19721728C2 (en) * | 1997-05-24 | 2001-07-12 | Byk Chemie Gmbh | Dispersants for pigments or fillers based on acrylic acid alkyl ester polymers, use and process for producing them |
| JPH11279408A (en) | 1997-06-02 | 1999-10-12 | Dainippon Ink & Chem Inc | Production of aqueous resin, aqueous curable resin composition and water-based coating material |
| US5985381A (en) | 1997-06-30 | 1999-11-16 | Conner; Kyle Henry | Methods for increasing a camouflaging effect and articles so produced |
| US5839718A (en) * | 1997-07-22 | 1998-11-24 | Usr Optonix Inc. | Long persistent phosphorescence phosphor |
| DE19732251B4 (en) * | 1997-07-26 | 2004-07-29 | Byk-Chemie Gmbh | Salinization products of polyamines and their use as dispersants for pigments and fillers |
| US6277037B1 (en) | 1997-10-03 | 2001-08-21 | Performance Dynamics Llc | Golf ball with water immersion indicator |
| US5823891A (en) | 1997-10-03 | 1998-10-20 | Performance Dynamics, Llc | Golf ball with water immersion indicator |
| US5914076A (en) | 1997-10-10 | 1999-06-22 | The Glo-Tech Corporation | Process for producing longer-lasting, high luminescence, phosphorescent textile fibers |
| US5833349A (en) | 1997-10-25 | 1998-11-10 | Apple; Wayne B. | Phosphorescent lamp shade |
| US6117362A (en) | 1997-11-07 | 2000-09-12 | University Of Georgia Research Foundation, Inc. | Long-persistence blue phosphors |
| JPH11140351A (en) * | 1997-11-07 | 1999-05-25 | Kowa Chem Ind Co Ltd | Water-based phosphorescent coating composition and method for applying same |
| US6267911B1 (en) | 1997-11-07 | 2001-07-31 | University Of Georgia Research Foundation, Inc. | Phosphors with long-persistent green phosphorescence |
| JPH11236524A (en) | 1998-02-20 | 1999-08-31 | Sakura Color Prod Corp | Phosphorescent ink composition and phosphorescent material |
| US6165234A (en) | 1998-03-26 | 2000-12-26 | Kanakkanatt; Sebastian V. | Thermally color-changing candles |
| US6096443A (en) * | 1998-07-17 | 2000-08-01 | Xerox Corporation | Transparencies |
| US6013122A (en) | 1998-08-18 | 2000-01-11 | Option Technologies, Inc. | Tattoo inks |
| US6130781A (en) | 1998-09-08 | 2000-10-10 | Gauvin; Aime H. | Skylight for day and night illumination |
| US6156325A (en) * | 1998-09-16 | 2000-12-05 | L'oreal | Nail enamel composition containing a urea-modified thixotropic agent |
| TWI285671B (en) | 1998-10-13 | 2007-08-21 | Orion 21 A D Pty Ltd | Luminescent gel coats and moldable resins |
| US6905634B2 (en) | 1998-10-13 | 2005-06-14 | Peter Burnell-Jones | Heat curable thermosetting luminescent resins |
| CA2356705A1 (en) | 1998-11-20 | 2000-06-02 | The General Hospital Corporation | Permanent, removable tissue markings |
| JP2000192034A (en) | 1998-12-25 | 2000-07-11 | Fuji Photo Film Co Ltd | Production of phosphor |
| JP4051645B2 (en) * | 1999-02-19 | 2008-02-27 | 有限会社アミティ | Textile products with phosphorescent reflectors |
| JP2000294130A (en) | 1999-04-08 | 2000-10-20 | Fujitsu Ltd | Phosphor layer forming method and plasma display panel |
| JP3803998B2 (en) | 1999-04-22 | 2006-08-02 | 横浜ゴム株式会社 | One-part moisture curable composition |
| EP1230579A1 (en) | 1999-05-18 | 2002-08-14 | Tutco, Inc. | Appliance windows coated with thermochromic polymer dispersed liquid crystal |
| US6623791B2 (en) | 1999-07-30 | 2003-09-23 | Ppg Industries Ohio, Inc. | Coating compositions having improved adhesion, coated substrates and methods related thereto |
| GB9918229D0 (en) | 1999-08-04 | 1999-10-06 | Ici Plc | Improvements relating to metal-compound catalysed processes |
| JP2001052866A (en) * | 1999-08-05 | 2001-02-23 | Fuji Electric Co Ltd | Fluorescence conversion filter and organic light- emitting element equipped with the filter |
| AU759971B2 (en) | 1999-09-29 | 2003-05-01 | Cygnet Works, Inc. | Thermochromic laminates and methods for controlling the temperature of a structure |
| US6400072B1 (en) | 2000-03-08 | 2002-06-04 | Motorola, Inc. | Viewing screen for a display device |
| DE10018581C1 (en) | 2000-04-14 | 2002-02-21 | Basf Coatings Ag | Color and / or effect painting with combination effect layer and their use |
| JP2001329047A (en) | 2000-05-25 | 2001-11-27 | Matsushita Electric Works Ltd | Epoxy resin composition, prepreg, resin adhered metal foil, adhesive sheet, and laminate |
| HUP0300802A3 (en) * | 2000-06-08 | 2005-07-28 | Lambert Stephen Hereford | Improved luminous materials |
| US6607744B1 (en) * | 2000-06-23 | 2003-08-19 | Segan Industries | Ingestibles possessing intrinsic color change |
| JP3547374B2 (en) | 2000-06-23 | 2004-07-28 | コナミ株式会社 | GAME SYSTEM AND STORAGE MEDIUM USED FOR THE SAME |
| US6617468B2 (en) * | 2000-08-16 | 2003-09-09 | Byk-Chemie Gmbh | Rheologically active urea urethane compounds |
| US20030022247A1 (en) * | 2000-10-03 | 2003-01-30 | Masabumi Shibuya | Substance which inhibits binding of information transfer molecule for 1175-tyrosine phosphorylated KDR/FLK-1 and usages of the same |
| US6499995B1 (en) | 2000-10-04 | 2002-12-31 | Dann A. Schwartz | Phosphorescent dental appliance and method of construction |
| EP1330499A2 (en) | 2000-10-11 | 2003-07-30 | Chemteall GmbH | Method for pretreating and subsequently coating metallic surfaces with a paint-type coating prior to forming and use of substrates coated in this way |
| JP2002234260A (en) | 2000-12-04 | 2002-08-20 | Pilot Ink Co Ltd | Reversible thermally discoloring and light transmissible laminate |
| US7163739B2 (en) | 2001-03-15 | 2007-01-16 | Mitsui Chemicals, Inc. | Laminate and display apparatus using the same |
| TW570876B (en) | 2001-05-11 | 2004-01-11 | Toyo Seikan Kaisha Ltd | Silicon oxide film |
| JP4017988B2 (en) | 2001-05-14 | 2007-12-05 | オムノバ ソリューソンズ インコーポレーティッド | Polymer surfactant derived from cyclic monomer having pendant fluorinated carbon group |
| US6800684B2 (en) | 2001-05-16 | 2004-10-05 | Toda Kogyo Corporation | Composite particles, and tread rubber composition, paint and resin composition using the same |
| DE10126652A1 (en) | 2001-06-01 | 2002-12-12 | Basf Coatings Ag | Coating powder, e.g. for painting cars, obtained by mixing liquid components in a static mixer, emulsifying with an aqueous solution of special emulsifier in a disperser, cooling, and isolating the solid particles |
| DE10126653A1 (en) | 2001-06-01 | 2002-12-12 | Basf Coatings Ag | Pigmented powder slurry, e.g. for painting cars, obtained by mixing liquid components in a static mixer, emulsifying with aqueous pigment suspension in a disperser and cooling the emulsion |
| US20030114562A1 (en) * | 2001-06-27 | 2003-06-19 | Pennzoil-Quaker State Company | Tinting composition and method of use |
| JP3608051B2 (en) * | 2001-07-27 | 2005-01-05 | ターンオン有限会社 | Liquefaction color light emission color storage material and method for producing the same |
| EP1288234A1 (en) | 2001-08-27 | 2003-03-05 | Sigma Coatings B.V. | Binders with low content in hydrolysable monomer suitable for selfpolishing antifouling paints |
| TW548683B (en) | 2001-10-23 | 2003-08-21 | Toray Industries | Dielectric paste and manufacturing method of plasma display |
| US6833191B2 (en) | 2001-11-20 | 2004-12-21 | Encap Technologies, Llc | Microencapsulated particles and process for manufacturing same |
| DE10156918A1 (en) | 2001-11-21 | 2003-06-05 | Ge Bayer Silicones Gmbh & Co | Paint-compatible to paintable polyorganosiloxane compositions |
| US6660184B2 (en) | 2001-12-13 | 2003-12-09 | Osram Sylvania Inc. | Phosphor paste compositions |
| US6750266B2 (en) | 2001-12-28 | 2004-06-15 | 3M Innovative Properties Company | Multiphoton photosensitization system |
| US20030222247A1 (en) * | 2002-01-28 | 2003-12-04 | Putman Everly Dean | Methods for manufacturing luminescent products having long afterglow |
| ES2288215T3 (en) * | 2002-05-08 | 2008-01-01 | Teijin Chemicals, Ltd. | COMPOSITION OF POLYCARBONATE RESIN, BALL OF THE SAME, AND MOLDED OBJECT OF THE SAME. |
| US20030219531A1 (en) | 2002-05-22 | 2003-11-27 | Farzad Parsapour | Method of manufacturing a dual color filter cathode ray tube (CRT) |
| ATE363716T1 (en) | 2002-06-14 | 2007-06-15 | Hyperion Catalysis Int | CARBON FIBRIL-BASED ELECTROCONDUCTIVE DYES AND COATINGS |
| US7050387B2 (en) * | 2002-06-28 | 2006-05-23 | Victor Company Of Japan, Ltd. | Optical storage medium |
| US7494704B2 (en) | 2002-08-15 | 2009-02-24 | Eastman Kodak Company | Material, article and method of preparing materials containing oriented anisotropic particles |
| US20040109853A1 (en) | 2002-09-09 | 2004-06-10 | Reactive Surfaces, Ltd. | Biological active coating components, coatings, and coated surfaces |
| US7241489B2 (en) | 2002-09-13 | 2007-07-10 | Jds Uniphase Corporation | Opaque flake for covert security applications |
| WO2004075624A2 (en) | 2003-02-24 | 2004-09-10 | Gnxpert Color, Inc. | System and methods for achieving signaling |
| US6953536B2 (en) | 2003-02-25 | 2005-10-11 | University Of Georgia Research Foundation, Inc. | Long persistent phosphors and persistent energy transfer technique |
| US20040187417A1 (en) | 2003-03-24 | 2004-09-30 | Thomas Paul J. | Changeable display system for the exterior of a house and ornaments for exterior window shutters |
| US6807909B1 (en) | 2003-06-19 | 2004-10-26 | William R. Coots | Method and apparatus for depositing railroad plates along a railroad track bed |
| US20050031838A1 (en) | 2003-08-06 | 2005-02-10 | Spectra Systems Corporation | Taggant security system for paper products as a deterrent to counterfeiting |
| GB0319639D0 (en) | 2003-08-21 | 2003-09-24 | Dodd Caroline M | Luminous container |
| KR100638157B1 (en) | 2003-09-04 | 2006-10-26 | 주고꾸 도료 가부시키가이샤 | Primary anti-corrosive paint composition and steel plate with primary anti-corrosive paint film |
| WO2005029163A1 (en) | 2003-09-17 | 2005-03-31 | Segan Industries, Inc. | Flash imaging devices, methods for making and using the same |
| KR20060090248A (en) | 2003-10-17 | 2006-08-10 | 디에스엠 아이피 어셋츠 비.브이. | Flame retardant uv cured buffered optical fibers and buffer composition |
| US20050197423A1 (en) * | 2003-11-07 | 2005-09-08 | Hidetoshi Fukuo | Photostorage solid drawing medium |
| US20050134164A1 (en) | 2003-12-18 | 2005-06-23 | 3M Innovative Properties Company | Optical coupler for projection display |
| TWI388876B (en) | 2003-12-26 | 2013-03-11 | Fujifilm Corp | Antireflection film, polarizing plate, method for producing them, liquid crystal display element, liquid crystal display device, and image display device |
| AU2004312516A1 (en) | 2003-12-30 | 2005-07-21 | General Electric Company | Polymer compositions, method of manufacture, and articles formed therefrom |
| US6990903B2 (en) | 2004-04-21 | 2006-01-31 | Print-Lock Corporation | Kit for labeling valuables for their identification and method therefor |
| US7138009B2 (en) * | 2004-06-22 | 2006-11-21 | Pitney Bowes Inc. | Signature protected photosensitive optically variable ink compositions and process |
| US20060159925A1 (en) | 2004-12-20 | 2006-07-20 | Satish Agrawal | High-intensity, persistent thermochromic compositions and objects, and methods for creating the same |
| US7910022B2 (en) * | 2006-09-15 | 2011-03-22 | Performance Indicator, Llc | Phosphorescent compositions for identification |
| US7547894B2 (en) * | 2006-09-15 | 2009-06-16 | Performance Indicator, L.L.C. | Phosphorescent compositions and methods for identification using the same |
-
2005
- 2005-12-20 US US11/311,289 patent/US20060159925A1/en not_active Abandoned
- 2005-12-20 AU AU2005319365A patent/AU2005319365B2/en not_active Ceased
- 2005-12-20 JP JP2007547016A patent/JP2008524401A/en active Pending
- 2005-12-20 US US11/311,290 patent/US20060172135A1/en not_active Abandoned
- 2005-12-20 MX MX2007007445A patent/MX2007007445A/en unknown
- 2005-12-20 WO PCT/US2005/046039 patent/WO2006069028A2/en active Application Filing
- 2005-12-20 US US11/793,376 patent/US8163201B2/en active Active
- 2005-12-20 SG SG201000375-4A patent/SG159492A1/en unknown
- 2005-12-20 CA CA 2591803 patent/CA2591803A1/en not_active Abandoned
- 2005-12-20 EP EP05854703A patent/EP1833943A4/en not_active Withdrawn
-
2010
- 2010-09-02 US US12/874,441 patent/US20110012062A1/en not_active Abandoned
-
2011
- 2011-08-03 US US13/115,843 patent/US8287757B2/en active Active
- 2011-08-05 US US13/115,850 patent/US8282858B2/en active Active
- 2011-08-05 US US13/115,854 patent/US8293136B2/en active Active
-
2012
- 2012-06-15 US US13/523,923 patent/US8409662B2/en active Active - Reinstated
Patent Citations (99)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2787558A (en) * | 1955-01-27 | 1957-04-02 | Firth Carpet Company Inc | Process of producing phosphorescent yarn |
| US3022189A (en) * | 1961-01-19 | 1962-02-20 | Du Pont | Daylight fluorescent coated fabric |
| US3508810A (en) * | 1967-07-19 | 1970-04-28 | Vari Light Corp | Photochromic systems |
| US3578602A (en) * | 1967-08-30 | 1971-05-11 | Fuji Photo Film Co Ltd | Photochromic compound |
| US3560211A (en) * | 1967-09-22 | 1971-02-02 | Horizons Research Inc | Light sensitive leuco dye systems containing no molecular oxygen therein |
| US3562172A (en) * | 1968-10-28 | 1971-02-09 | Fuji Photo Film Co Ltd | Photochromic compound and composition containing the same |
| US3884697A (en) * | 1969-03-31 | 1975-05-20 | Eiichi Inoue | Photographic process utilizing spiropyran compound dispersed in nitrocellulose films with high nitrogen content |
| US3650812A (en) * | 1969-12-24 | 1972-03-21 | Ford Motor Co | Acrylic-siloxane resin paint and painted article |
| US3666352A (en) * | 1970-01-22 | 1972-05-30 | Charles A Wagner | Rate controlled photochromic lenses of vinyl chloride-vinyl acetate copolymer containing a mercury thiocarbazone compound |
| US3654190A (en) * | 1970-05-28 | 1972-04-04 | Us Navy | Fire retardant intumescent paint |
| US3668189A (en) * | 1970-08-24 | 1972-06-06 | Allied Chem | Fluorescent polycarbonamides |
| US3714181A (en) * | 1970-12-31 | 1973-01-30 | American Cyanamid Co | 2-aryl-5,10-diphenylphenanthro(9,10-d)azoles |
| US3936970A (en) * | 1971-05-10 | 1976-02-10 | Hodges John A | Fishing lure and method of fishing |
| US4028118A (en) * | 1972-05-30 | 1977-06-07 | Pilot Ink Co., Ltd. | Thermochromic materials |
| US3738299A (en) * | 1972-06-22 | 1973-06-12 | M Packler | Emblems which will glow in the dark and the method of making them |
| US3873390A (en) * | 1972-09-27 | 1975-03-25 | Richard K Cornell | Phosphorescent, fluorescent and reflective coated sheets or films and compositions and method for making the same |
| US4025661A (en) * | 1972-11-13 | 1977-05-24 | Rca Corporation | Method of making viewing-screen structure for a cathode-ray tube |
| US3957678A (en) * | 1973-01-11 | 1976-05-18 | U.S. Philips Corporation | Method of manufacturing a luminescent sulfide |
| US4208300A (en) * | 1973-07-11 | 1980-06-17 | Gravisse Philippe E | Photoluminescent materials and method of manufacturing same |
| US4567019A (en) * | 1977-05-11 | 1986-01-28 | Graphic Controls Corporation | Color reversing compositions |
| US4188449A (en) * | 1977-08-04 | 1980-02-12 | Eastman Kodak Company | Phosphorescent screens |
| US4375373A (en) * | 1978-12-29 | 1983-03-01 | Toro Ganryo Kogyo Co., Ltd. | Method of coating inorganic pigments (ultramarine and bronze powder) with dense amorphous silica |
| US4268134A (en) * | 1979-03-07 | 1981-05-19 | Corning Glass Works | Lightweight laminated photochromic lenses |
| US4425161A (en) * | 1980-11-27 | 1984-01-10 | Yutaka Shibahashi | Thermochromic materials |
| US4379100A (en) * | 1981-02-02 | 1983-04-05 | Ex-Cell-O Corporation | Polyurethane molding process with siloxane internal release agent |
| US4425377A (en) * | 1981-07-22 | 1984-01-10 | Rca Corporation | Method of making a cathode-ray tube having a conductive internal coating exhibiting reduced arcing current |
| US4440672A (en) * | 1982-03-22 | 1984-04-03 | American Optical Corporation | Photochromic composition resistant to fatigue |
| US4720356A (en) * | 1982-03-22 | 1988-01-19 | American Optical Corporation | Photochromic composition resistant to fatigue |
| US4451504A (en) * | 1983-05-20 | 1984-05-29 | North American Philips Consumer Electronics Corp. | Process for applying phosphor to the aperture mask of a cathode ray tube |
| US4637698A (en) * | 1983-11-04 | 1987-01-20 | Ppg Industries, Inc. | Photochromic compound and articles containing the same |
| US4663214A (en) * | 1985-01-04 | 1987-05-05 | Coburn Jr Joseph W | Phosphorescent material and process of manufacture |
| US4734295A (en) * | 1985-01-07 | 1988-03-29 | Liu P Dong Guang | Glare control |
| US4717710A (en) * | 1985-01-17 | 1988-01-05 | Matsui Shikiso Chemical Co. Ltd. | Thermochromic composition |
| US4830875A (en) * | 1985-10-10 | 1989-05-16 | Quantex Corporation | Photoluminescent materials and associated process and infrared sensing device |
| US4826550A (en) * | 1985-11-28 | 1989-05-02 | Matui Shikiso Chemical Co., Ltd. | Process for preparing molded product of thermochromic polyvinyl chloride |
| US4717770A (en) * | 1986-02-27 | 1988-01-05 | Sumitomo Chemical Co., Ltd. | Process for producing epsilon-caprolactam |
| US4913544A (en) * | 1986-05-01 | 1990-04-03 | Pilkington Plc | Photochromic articles |
| US4826977A (en) * | 1986-05-15 | 1989-05-02 | The Plessey Company Plc | Photochromic spiropyran compounds |
| US4818096A (en) * | 1986-06-17 | 1989-04-04 | The Plessey Company Plc | Photoreactive lenses with adamantane spiro compounds |
| US4910252A (en) * | 1986-07-07 | 1990-03-20 | Kansai Paint Co., Ltd. | Siloxane polymer antifouling paint composition containing polysiloxanes |
| US4927180A (en) * | 1986-08-22 | 1990-05-22 | Plessey Overseas Limited | Marking of articles with photochromic compounds |
| US4898895A (en) * | 1986-12-30 | 1990-02-06 | Nippon Oil And Fats Co., Ltd. | Antifouling pain having a polyacrylate component with pendent silyl or siloxane groups |
| US4729907A (en) * | 1987-02-24 | 1988-03-08 | Rca Corporation | Method of making a viewing screen structure for a cathode-ray tube |
| US5630869A (en) * | 1988-01-12 | 1997-05-20 | Sicipa Holding S.A. | Reversibly photochromic printing inks |
| US4921727A (en) * | 1988-12-21 | 1990-05-01 | Rca Licensing Corporation | Surface treatment of silica-coated phosphor particles and method for a CRT screen |
| US5605734A (en) * | 1989-11-02 | 1997-02-25 | Basf Corporation | Phosphorescent directional signals and manufacturing method |
| US5176905A (en) * | 1989-11-30 | 1993-01-05 | Shiseido Co., Ltd. | Photochromic flesh-colored pigment and process for producing the same |
| US5007647A (en) * | 1989-12-15 | 1991-04-16 | Sports Glow, Inc. | Golf ball and method of making same |
| US5023015A (en) * | 1989-12-19 | 1991-06-11 | Gte Products Corporation | Method of phosphor preparation |
| US5185390A (en) * | 1990-03-07 | 1993-02-09 | Ppg Industries, Inc. | Water strippable photochromic resin composition |
| US5221288A (en) * | 1990-10-09 | 1993-06-22 | Matsui Shikiso Chemical Co., Ltd. | Thermochromic dyeing method and cellulose product dyed thereby |
| US5223330A (en) * | 1990-11-28 | 1993-06-29 | Precision Fabrics Group, Inc. | Phosphorescent fiber reinforced plastic article and process for making the same |
| US5387458A (en) * | 1990-12-06 | 1995-02-07 | Minnesota Mining And Manufacturing Company | Articles exhibiting durable fluorescence with an ultraviolet screening layer |
| US5219625A (en) * | 1991-07-09 | 1993-06-15 | The Pilot Ink Co., Ltd. | Thermochromic laminate member and toy utilizing the same |
| US5294375A (en) * | 1991-08-20 | 1994-03-15 | Polaroid Corporation | Thermochromic materials |
| US5480482A (en) * | 1991-11-04 | 1996-01-02 | The United States Of America As Represented By The Secretary Of The Navy | Reversible thermochromic pigments |
| US5389093A (en) * | 1992-04-01 | 1995-02-14 | Howell; Wesley A. | Wetness indicating diaper |
| US6391492B1 (en) * | 1992-04-05 | 2002-05-21 | Canon Kabushiki Kaisha | Secondary battery |
| US5395673A (en) * | 1992-04-23 | 1995-03-07 | Hunt; Gary B. | Non-slip surface |
| US5391327A (en) * | 1992-09-25 | 1995-02-21 | Transitions Optical, Inc. | Photochromic compositions of improved fatigue resistance |
| US5292549A (en) * | 1992-10-23 | 1994-03-08 | Armco Inc. | Metallic coated steel having a siloxane film providing temporary corrosion protection and method therefor |
| US5321069A (en) * | 1992-11-25 | 1994-06-14 | Afterglow Accent Yarns, Inc. | Process for producing phosphorescent yarn and yarn produced by the process |
| US5618063A (en) * | 1992-12-09 | 1997-04-08 | Wallace Computer Services, Inc. | Multicolor heat-sensitive verification and highlighting system |
| US5378897A (en) * | 1992-12-09 | 1995-01-03 | Fuji Photo Film Co., Ltd. | Radiation image storage panel |
| US6060428A (en) * | 1992-12-09 | 2000-05-09 | Wallace Computer Services, Inc. | Heat-sensitive chromogenic system |
| US5427415A (en) * | 1992-12-09 | 1995-06-27 | Wallace Computer Services, Inc. | Heat sensitive system and use thereof |
| US5424006A (en) * | 1993-04-28 | 1995-06-13 | Nemoto & Co., Ltd. | Phosphorescent phosphor |
| US5728758A (en) * | 1993-12-13 | 1998-03-17 | Transitions Optical, Inc. | Coating composition and articles having a cured coating |
| US5490344A (en) * | 1994-03-22 | 1996-02-13 | Bussiere; Robert A. | Glow-in-the-dark material for fishing accessories |
| US5744233A (en) * | 1994-09-09 | 1998-04-28 | U.S. Philips Corporation | Method of coating luminescent powders, luminescent powders and coated object |
| US6027810A (en) * | 1994-10-07 | 2000-02-22 | Minnesota Mining & Manufacturing | Radiographic intensifying screen with antistat |
| US5731658A (en) * | 1994-11-30 | 1998-03-24 | Honeywell Inc. | Ultraviolet binder for phosphor fluorescent light box |
| US5607621A (en) * | 1994-12-28 | 1997-03-04 | Ykk Corporation | Phosphorescent synthetic resin material method for production thereof, and formed article |
| US5717282A (en) * | 1995-02-20 | 1998-02-10 | U.S. Philips Corporation | Display device comprising a display screen having a light-absorbing coating |
| US5708184A (en) * | 1995-07-31 | 1998-01-13 | Bayer Aktiengesellschaft | Process for the preparation of substituted aminocarbonyltriazolinones |
| US6048347A (en) * | 1995-11-01 | 2000-04-11 | Micro Medical Devices, Inc. | Lens storage and folding apparatus |
| US6177487B1 (en) * | 1995-11-03 | 2001-01-23 | Basf Coatings Ag | Aqueous powder paint dispersions |
| US5716723A (en) * | 1996-03-07 | 1998-02-10 | Van Cleef; James Gresham | Glow in the dark shoe sole |
| US5753146A (en) * | 1996-03-29 | 1998-05-19 | Transitions Optical, Inc. | Photochromic naphthopyran compositions of neutral color |
| US5770115A (en) * | 1996-04-19 | 1998-06-23 | Ppg Industries, Inc. | Photochromic naphthopyran compositions of improved fatigue resistance |
| US6344233B1 (en) * | 1996-10-15 | 2002-02-05 | Institute For Radiological Image Sciences, Inc. | Method of producing a phosphor screen |
| US5730961A (en) * | 1997-01-24 | 1998-03-24 | Goudjil; Kamal | Metamorphic nail polish |
| US6878076B2 (en) * | 1997-10-03 | 2005-04-12 | Performance Indicator, Llc | Golf ball with moisture exposure indicator |
| US6358160B1 (en) * | 1997-10-03 | 2002-03-19 | Performance Dynamics Llc | Golf ball with water immersion indicator |
| US6046455A (en) * | 1998-01-30 | 2000-04-04 | Segan Industries | Integrating ultraviolet exposure detection devices |
| US6201057B1 (en) * | 1998-02-23 | 2001-03-13 | Therma-Tru Corporation | Weatherable coating and stain system for thermoset or thermoplastic composite surfaces |
| US6375864B1 (en) * | 1998-11-10 | 2002-04-23 | M.A. Hannacolor, A Division Of M.A. Hanna Company | Daylight/nightglow colored phosphorescent plastic compositions and articles |
| US6196241B1 (en) * | 1999-05-19 | 2001-03-06 | Denise Doolan | Color changing umbrella |
| US6359048B1 (en) * | 1999-06-05 | 2002-03-19 | Van Duynhoven Debra May | Tintable luminescent paint |
| US6553696B1 (en) * | 1999-09-17 | 2003-04-29 | Robert Foster, Sr. | Flourescent drink rim |
| US6207077B1 (en) * | 2000-02-18 | 2001-03-27 | Orion 21 A.D. Pty Ltd | Luminescent gel coats and moldable resins |
| US6508732B1 (en) * | 2000-07-03 | 2003-01-21 | Mildred Kinghorn Romberger | Tennis ball |
| US6710127B2 (en) * | 2000-09-29 | 2004-03-23 | Byk- Chemie Gmbh | Levelling agents for surface coatings |
| US6894124B2 (en) * | 2000-11-01 | 2005-05-17 | Kansai Paint Co., Ltd. | High solid paint compositions |
| US6514594B1 (en) * | 2000-11-09 | 2003-02-04 | Avery Dennison Corporation | Fluorescent polymeric articles having screening layer formed from U.V. light absorbing polymer |
| US6861467B2 (en) * | 2002-06-28 | 2005-03-01 | Okitsumo Incorporated | Powder coating composition |
| US20040009833A1 (en) * | 2002-07-10 | 2004-01-15 | Ja-Ru, Inc. | Glow-in-the-dark wrist toy |
| US6870024B2 (en) * | 2002-09-09 | 2005-03-22 | Byk-Chemie Gmbh | Polymeric urea-urethane rheology control agents and a process for their preparation |
| US7033712B2 (en) * | 2003-01-30 | 2006-04-25 | Thomson Licensing | Method of manufacturing a color filter cathode ray tube (CRT) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20120080615A1 (en) * | 2010-09-30 | 2012-04-05 | Performance Indicator, Llc. | Photolytically and environmentally stable multilayer structure for high efficiency electromagentic energy conversion and sustained secondary emission |
| US8232533B2 (en) * | 2010-09-30 | 2012-07-31 | Performance Indicator, Llc | Photolytically and environmentally stable multilayer structure for high efficiency electromagnetic energy conversion and sustained secondary emission |
| CN110791190A (en) * | 2019-10-30 | 2020-02-14 | 湖南松井新材料股份有限公司 | Water-based self-luminous elastic coating and preparation method and application thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2006069028A3 (en) | 2006-10-05 |
| AU2005319365A1 (en) | 2006-06-29 |
| US20120021251A1 (en) | 2012-01-26 |
| US20060172135A1 (en) | 2006-08-03 |
| AU2005319365B2 (en) | 2011-02-03 |
| US20120027930A1 (en) | 2012-02-02 |
| US20080185557A1 (en) | 2008-08-07 |
| US8409662B2 (en) | 2013-04-02 |
| WO2006069028A2 (en) | 2006-06-29 |
| SG159492A1 (en) | 2010-03-30 |
| CA2591803A1 (en) | 2006-06-29 |
| US20120028054A1 (en) | 2012-02-02 |
| EP1833943A4 (en) | 2011-09-28 |
| US20110012062A1 (en) | 2011-01-20 |
| MX2007007445A (en) | 2007-11-08 |
| EP1833943A2 (en) | 2007-09-19 |
| US8287757B2 (en) | 2012-10-16 |
| US20120251712A1 (en) | 2012-10-04 |
| US8163201B2 (en) | 2012-04-24 |
| JP2008524401A (en) | 2008-07-10 |
| US8293136B2 (en) | 2012-10-23 |
| US8282858B2 (en) | 2012-10-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20060159925A1 (en) | High-intensity, persistent thermochromic compositions and objects, and methods for creating the same | |
| EP0677564B1 (en) | Opaque/transparent thermochromic compositions and laminate | |
| US5721059A (en) | Temperature-dependent color/transparency storing resin composition and laminate member employing the same | |
| JP3396787B2 (en) | Thermochromic color memory microcapsule pigment | |
| KR100689001B1 (en) | Fluorescent red composition and articles made therefrom | |
| JP2007098778A (en) | Reversible thermally discoloring transfer sheet, and reversible thermally discoloring article using it | |
| JP4347749B2 (en) | Thermosensitive color-change color memory laminate | |
| KR200417096Y1 (en) | transfer paper | |
| JP4934255B2 (en) | Light-resistant thermochromic material | |
| JP2015024582A (en) | Method of arranging adhesive layer on reversible thermochromic laminate and reversible thermochromic pasted body | |
| JP3333912B2 (en) | Luminescent ink and luminous printed matter using the same | |
| KR100782282B1 (en) | Method made transfer paper | |
| JPH09309174A (en) | Thermal discoloring laminated body | |
| JP5955666B2 (en) | Drawing set and drawing method using the same | |
| JP2008207510A (en) | Brilliant thermal-discoloring laminate | |
| JP2004322423A (en) | Metallic gloss tone thermodiscoloring laminate | |
| JP2012188796A (en) | Method for manufacturing rugged color-changing fabric | |
| JPH11208194A (en) | Printed matter for transfer | |
| JP2015009369A (en) | Reversible thermochromic laminate and reversible thermochromic pastable body | |
| JP2003127293A (en) | Metallic gloss tone thermally discoloring laminate | |
| JPH0834157A (en) | Light accumulation type thermal color-changeable laminate | |
| JP2006034320A (en) | Light-storing and reversibly thermochromic toy | |
| JP2012187910A (en) | Corrugated metachromatic fabric, and method of manufacturing the same | |
| JP2012106502A (en) | Light-resistant heat-fading body |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: PERFORMANCE INDICATOR LLC, MASSACHUSETTS Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:AGRAWAL, SATISH;OSINSKI, ROBB J.;WINSKOWICZ, ROBERT;AND OTHERS;REEL/FRAME:017386/0081;SIGNING DATES FROM 20060307 TO 20060308 |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |