IMATINIB MESYLATE CRYSTAL FORM AND PROCESS FOR PREPARATION THEREOF
The present invention provides imatinib mesylate in a crystalline form and a process for preparation thereof. In one aspect the present invention provides a non-needle shaped a- crystalline form of imatinib mesylate. In another aspect the present invention provides crystalline form of imatinib mesylate characterized in that the difference between the tapped and untapped density is less than 0.15 gm/ml.
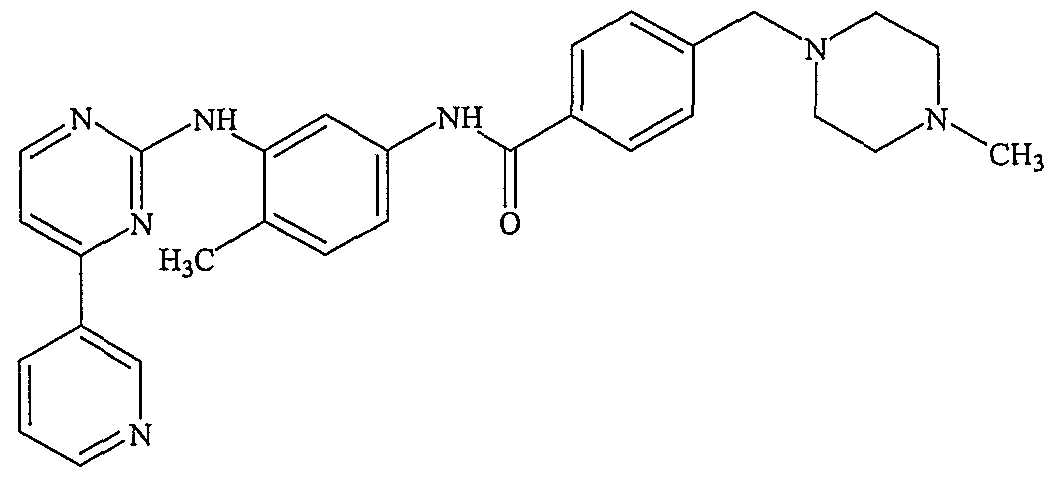
The present invention also provides a process for the preparation of a crystalline form of imatinib mesylate. Particularly, the present invention provides a novel process for the preparation of a non-needle shaped α-crystalline form of imatinib mesylate, a methane sulfonic acid addition salt of 4-[(4-methyl-l-piperazinyl)methyl]-N-[4-methyl-3-[[4-(3- pyridinyl)-2-pyrimidinyl] amino] -phenyl]benzamide of formula 1. The mesylate salt of Imatinib (Gleevec®) has been approved for the treatment of Chronic Myeloid Leukemia.
Formula 1
BACKGROUND OF THE INVENTION
United States Patent No. 5521184 discloses N-phenyl-2-pyrimidine amine compounds including the compound of formula 1.
United States Patent No. 6894051 (equivalent of WO 99/03854) discloses that the methanesulfonic acid addition salt of imatinib (imatinib mesylate) can exist in needle- shaped α-crystalline form or non-needle-shaped β-crystalline form. It is reported that the
α-crystalline form of imatinib mesylate is characterized by needle-shaped crystals, is hygroscopic and not particularly well suited to pharmaceutical formulation as solid dosage forms because of its physical properties, for example its flow characteristics are unfavourable. The patent application discloses a method for preparation of the a- crystalline form of imatinib mesylate wherein a hot solution of imatinib mesylate in aqueous ethanol is cooled. However, we have found that this process for preparation of the α-crystalline form is inconsistent, non-reproducible. In order to overcome the drawbacks of the α-crystalline form, the patent application discloses the /3-crystalline form of imatinib mesylate and the process for its preparation.
We have found surprising results when we prepared crystalline imatinib mesylate by the process of thin film drying. We found that the process resulted in a crystalline imatinib mesylate in a non-needle shaped form. The process resulted in a crystalline form that has a bulk density, which is relatively insensitive to tapping and which is non-hygroscopic. This crystalline imatinib mesylate is easy to handle and convenient to process into a dosage forms, for example it can be conveniently formulated and processed into tablets by dry granulation and direct compression methods.
We have also found a process for preparation of imatinib mesyalte in α-crystalline form in a reproducible manner, which is convenient for industrial use to provide α-crystalline form of imatinib mesylate reproducibly.
Definitions:
As used herein, "particle size distribution" means the distribution of equivalent spherical diameters.
The term XN as used herein denotes the particle size in microns (μm) below which there are N% of particles.
As used herein "aspect ratio" is the ratio between the mean length and the mean width of the crystals.
Figure 1 provides X-ray diffractogram of the α-crystalline form of imatinib mesylate prepared according to the process of the present invention.
Figure 2 provides infrared spectrum (IR) of the α-crystalline form of imatinib mesylate prepared according to the process of the present invention.
Figure 3 provides a differential scanning thermogram (DSC) of the ce-crystalline form of imatinib mesylate prepared according to the process of the present invention.
Figure 4 provides an optical photograph of the α-crystalline form of imatinib mesylate prepared according to the process of the present invention.
Figure 5 provides an optical photograph of the /3-crystalline form of imatinib mesylate.
SUMMARY OF THE INVENTION
The present invention provides crystalline imatinib mesylate in a non-needle shaped a- crystalline form.
m a preferred embodiment the present invention provides crystalline imatinib mesylate in a non-needle shaped α-crystalline form having an aspect ratio between the range of about 1 to about 2, more preferably between the range of about 1 to about 1.5.
In another aspect the present invention provides a crystalline form of imatinib mesylate characterized in that the difference between the tapped and untapped density is less than 0.15 gm/ml.
In another aspect the present invention provides a crystalline form of imatinib mesylate characterized in that the water uptake is not more than 1.0% w/w, preferably not more than 0.6% w/w at 80% RH over 90 hours.
The present invention provides a process for the preparation of the ce-crystalline form of imatinib mesylate, a methane sulfonic acid addition salt of 4-[(4-methyl-l- piperazinyl)methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-phenyl] benzamide of formula 1,
Formula 1 comprising subjecting a solution of imatinib mesylate in a solvent to thin film drying.
In preferred embodiment, the present invention provides a process for preparation of the α-crystalline form of imatinib mesylate, comprising subjecting a solution of imatinib mesylate in a polar protic solvent to agitated thin film drying.
DETAILED DESCRIPTION OF THE INVENTION
The crystalline form of imatinib mesylate of the present invention is non-needle shaped and has good flow properties. Generally, the aspect ratio i.e. the ratio of mean length and mean width of the crystals may be between the range of about 1 to about 3, preferably about 1 to about 2, more preferably about 1 to about 1.5. An interesting property found with the crystalline imatinib mesylate of the present invention is that the difference between the tapped and untapped density is less than 0.15 gm/ml. Without ascribing to any theory the reason for this is perhaps that the particles get distributed such that the finer particles occupy the spaces between the coarser particles so that the particles become densely packed, making the material non-fluffy, and having better flow properties. This results in the imatinib mesylate bulk that has good compressibility and hence convenient to process into a dosage form. Also the crystalline form of imatinib
mesylate of the present invention is having water uptake not more than 1.0% w/w, preferably not more than 0.6% w/w at 80% RH over 90 hours.
The present invention provides crystalline imatinib mesylate in a non-needle shaped a- crystalline form.
In one preferred embodiment the present invention provides crystalline imatinib mesylate in a non-needle shaped α-crystalline form having an aspect ratio between the range of about 1 to about- 2.
In another preferred embodiment the present invention provides crystalline imatinib mesylate in a non-needle shaped α-crystalline form having an aspect ratio between the range of about 1 to about 1.5.
In another preferred embodiment present invention provides crystalline imatinib mesylate in a non-needle shaped α-crystalline form characterized in that the particle size distribution is such that the ratio of XioiXso÷Xθo is in the range of 1: (2 to 8):(5 to 20), wherein X10, X50 and X90 represent the sizes below which there are 10%, 50% and 90% of the particles, respectively.
In another aspect the present invention provides crystalline imatinib mesylate characterized in that the difference between the tapped and untapped density is less than 0.15 gm/ml.
In one preferred embodiment present invention provides crystalline form of imatinib mesylate characterized in that the particle size distribution is such that the ratio of X10:X50:X9o is in the range of 1: (2 to 8):(5 to 20), wherein X10, X50 and X90 represent the sizes below which there are 10%, 50% and 90% of the particles, respectively.
In another preferred embodiment present invention provides crystalline imatinib mesylate in a non-needle shaped α-crystalline form characterized in that the difference between the tapped and untapped density is less than 0.15 gm/ml.
The present invention also provides novel, simple and viable process for the preparation of α-crystalline form of imatinib mesylate in a consistent manner, by subjecting a solution of imatinib mesylate to thin film drying.
The solution of imatinib mesylate to be subjected to thin film drying for preparation of the crystalline form may be prepared in a suitable solvent. The suitable solvent to prepare a solution of imatinib mesylate may be a polar protic or aprotic solvent, a non-polar solvent, water or mixture thereof.
A person of skill in the art is familiar with the terms polar protic or aprotic or a non-polar solvent. A reference is made to Chapter 3, Classification of solvents, "Solvents and Solvent effects in Organic Chemistry" second edition, Christian Reichardt, © VCH, 1988.
Preferably a polar protic water miscible solvent may be used. For example, the polar protic solvent is an alcohol or aqueous alcohol solvent, for example, methanol, ethanol, isopropanol, n-butanol, iso-butanol, tert-butanol, water or mixture thereof. More preferably, the solvent is methanol and water.
The solution of imatinib mesylate to be subjected to thin film drying may be advantageously prepared using a polar protic water miscible solvent and water mixture, comprising 0 to 80% v/v, preferably 10 to 50% v/v and more preferably 15 to 30% v/v of the said polar protic solvent.
The concentration of imatinib mesylate in the solvent to be subjected to thin film drying may be up to 30% w/v, preferably 3 to 15% and more preferably 5 to 10% w/v.
The temperature at which the solution of imatinib mesylate is subjected to thin film drying may be ambient to about 90°C.
The flow rate may be adjusted such that from a solution of imatinib mesyalte subjected to thin film drying, the rate of drying the solvent may be 20L/hour, advantageously 10L/hour at temperature of about 60°C.
The time for which the process of the present invention may be carried out is variable, generally about 1 to about 12 hours depending on the factors like temperature, solvent, flow rate etc would be adequate. For a batch of about 2 Kg quantity in a solvent like methanol and water, generally about 2 to about 3 hours would be sufficient at vapour temperature of about 600C with flow rate of about 10 to about 20L/hour.
The starting imatinib mesylate that is dissolved in a solvent to prepare a solution of imatinib mesylate may be imatinib mesylate in any form for example, a β-crystalline form, amorphous imatinib mesylate or mixture thereof. It can also be a mixture of α- crystalline form with β-crystalline form or amorphous form thereof. The starting imatinib mesylate may be prepared by any method known in the literature.
hi the process of the present invention to prepare ocrystalline form of imatininb mesylate, a solution of imatinib mesylate in a suitable solvent can be subjected to agitated thin film drying (ATFD) under atmospheric pressure and/or under vacuum.
The process of the present invention in one aspect provides crystalline imatinib mesylate in a non-needle shaped ce-crystalline form.
hi a preferred embodiment the process of the present invention provides crystalline imatinib mesylate in a non-needle shaped α-crystalline form having an aspect ratio between the range of about 1 to about 3.
In one preferred embodiment the process of the present invention provides crystalline imatinib mesylate in a non-needle shaped α-crystalline form having an aspect ratio between the range of about 1 to about 2.
In another preferred embodiment the process of the present invention provides crystalline imatinib mesylate in a non-needle shaped α-crystalline form having an aspect ratio between the range of about 1 to about 1.5.
The process of the present invention in another aspect provides crystalline imatinib mesylate characterized in that the difference between the tapped and untapped density is less than 0.15 gm/ml.
This crystalline form of imatinib mesylate is characterized in that the particle size distribution is such that the ratio of XiO:X5O:X9o is in the range of 1 : (2 to 8):(5 to 20), wherein X10, X50 and X90 represent the sizes below which there are 10%, 50% and 90% of the particles, respectively.
The crystalline form of imatinib mesylate of the present invention can be prepared conveniently by following the process of the present invention by subjecting a solution of imatinib mesylate in a solvent to thin film drying.
In a preferred embodiment the imatinib mesylate in α-crystalline form is prepared by subjecting a solution of imatinib mesylate in methanol and water to thin film drying at vapor temperature about 50°C to about 70°C under vacuum.
In another preferred embodiment the imatinib mesylate in α-crystalline form is prepared by subjecting a solution of imatinib mesylate in methanol and water to thin film drying at vapor temperature of about 50°C to about 70°C, optionally under vacuum, wherein methanol:water is used between the range of 1 :4 to 1 : 1 (v/v) ratio.
In another preferred embodiment the imatinib mesylate in α-crystalline form is prepared by subjecting a solution of imatinib mesylate in methanol and water to thin film drying at vapor temperature of about 500C to about 70°C, optionally under vacuum, wherein methanol and water used is 1:1 (v/v) ratio.
In another preferred embodiment the imatinib mesylate in α-crystalline form is prepared by subjecting a solution of imatinib mesylate in methanol and water to thin film drying at vapor temperature of about 50°C to about 7O0C, optionally under vacuum, wherein methanol:water used is 1 :4 (v/v) ratio.
The crystalline form of imatinib mesylate of the present invention has good compressibility and can be used for preparing tablets by direct compression or dry granulation. The tablets prepared by using the crystalline form of imatinib mesylate prepared according to process of the present invention have low friability, good surface finish and are less prone to abrasion.
The examples that follow do not limit the scope of the present invention and are merely used as illustrations.
EXAMPLES
Example 1
Preparation of α-form of imatinib mesylate:
Imatinib mesylate (2.0 Kg) is dissolved in a mixture of Methanol: water (6 Lit : 24 Lit.) at 25-30°C temperature to get a clear solution. The solution was concentrated in agitated thin film drier (ATFD) with flow rate of about 18 to 20 L per hour using Peristaltic pump at vapor temperature of about 55-600C under vacuum for 90 minutes. The solid obtained was dried at 60-750C under vacuum for 60-90 minutes. The product obtained is α- crystalline form of imatinib mesylate (weight: 1.4-1.5 Kg).
Example 2
Preparation of α-form of imatinib mesylate: rmatinib mesylate (500 gm) is dissolved in a mixture of Methanol:water (1:1, 5.0 L) at 25-3O0C temperature to get a clear solution. The solution was concentrated in agitated thin film drier (ATFD) with flow rate of about 10 to 12 L per hour using Peristaltic pump at vapor temperature of about 50-550C under vacuum for 30 minutes. The solid obtained was dried at 50-550C under vacuum for 30-45 minutes. The product obtained is α- crystalline form of imatinib mesylate (weight: 430.0 gm).
Given below is the data recorded for three batches of α-crystalline form of imatinib mesylate prepared according to process of the present invention.
Density:
In contrast, it was found that the difference in tapped and untapped densities of the /S- crystalline form of imatinib mesylate was in the range of 0.17 to 0.26.
Particle size distribution data:
Particle size distribution data recorded by laser diffraction in a Helos Symaptec analyzer, using cyclohexane as a dispersant with sonication duration of 10.00 seconds for three batches of α-crystalline form of imatinib mesylate prepared according to process of the present invention is:
wherein X10, X16, Xs0, X84, X90 and X99 represent the sizes below which there are 10%, 16%, 50%, 84%, 90% and 99% of the particles, respectively.
The table below provides the data recorded for ce-crystalline form of imatinib mesylate of the present invention compared to the known β-crystalline form of imatinib mesylate at 80% relative humidity (RH) over 90 hours.
It is apparent that the α-crystalline form of imatinib mesylate prepared by the process of the invention is stable and the % weigh gain in moisture is not more than 0.6% at 80%RH over 90 hours.
Aspect Ratio:
Aspect ratio data recorded on Nikon microscope using 250 counts for three batches of ce- crystalline form of imatinib mesylate prepared according to process of the present invention is:
Example 3:
Tablets of imatinib mesylate (100 mg) prepared according to thin film drying process
The Stage A ingredients are sifted through # 60 mesh s.s. screen and granulated using isopropyl alcohol, milled and the wet mass is passed through 10mm s.s. screen in comminuting mill. The granules are dried in fluidized bed drier at 60°C inlet
temperature till loss on drying in Halogen Moisture balance at 105°C constant weight is l-2%w/w. The dried granules are milled through 1.5mm s.s. screen in comminuting mill and transferred to a blender. The Stage C ingredients are sifted through # 60 mesh s.s. screen and transferred to the blender. The granules are lubricated in the blender for 10 minutes. The tablets are compressed at tablet weight of 290mg using 9.5mm, circular, DR punches and film coated with Stage D ingredients in a suitable coating machine.